Key Points
-
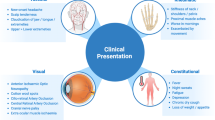
Visual loss is the most feared manifestation of giant cell arteritis (GCA) and occurs in up to 20% of patients before glucocorticoid therapy is commenced
-
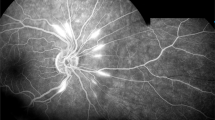
Anterior ischaemic optic neuropathy (AION) owing to arteritis of the posterior ciliary arteries is the most common cause of visual loss in GCA and must be differentiated from non-arteritic AION
-
Cerebrovascular accidents — stroke and transient ischaemic attack — occur in 1.5–7% of patients with GCA and are caused by stenosis or occlusion of the extradural vertebral or carotid arteries
-
A previous ischaemic event in GCA is the strongest predictor for a subsequent event; patients with traditional cardiovascular risk factors and a lower inflammatory response are more likely to develop ischaemic manifestations
-
Adequate doses of glucocorticoids in GCA largely prevent further cranial ischaemic events, but are scarcely effective at improving established visual loss
-
Fast-track clinics for the diagnosis of GCA might substantially reduce the occurrence of permanent sight loss by reducing diagnostic delay.
Abstract
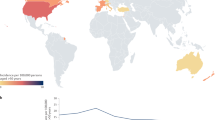
Giant cell arteritis (GCA) is the most common form of vasculitis in individuals aged 50 years and over. GCA typically affects large and medium-sized arteries, with a predilection for the extracranial branches of the carotid artery. Patients with GCA usually present with symptoms and signs that are directly related to the artery that is affected, with or without constitutional manifestations. The most dreaded complication of GCA is visual loss, which affects about one in six patients and is typically caused by arteritis of the ophthalmic branches of the internal carotid artery. Before the advent of glucocorticoid treatment, the prevalence of visual complications was high. Increasing awareness by physicians of the symptoms of GCA and advances in diagnostic techniques over the past twenty years have also contributed to a substantial decline in the frequency of permanent visual loss. Ischaemic brain lesions are less common than visual lesions, and mostly result from vasculitis of the extradural vertebral or carotid arteries. In the case of both the eye and the brain, ischaemic damage is thought to result from arterial stenosis or occlusion that occurs secondary to the inflammatory process. The inflammatory response at the onset of arteritis, its role as a predictor of complications and the role of traditional cardiovascular risk factors have been extensively investigated in the past decade. In this Review, the epidemiology, risk factors, clinical presentation and current therapeutic approach of GCA-related ischaemic events are discussed, with a particular emphasis on visual loss.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gonzalez-Gay, M. A., Castaneda, S. & Llorca, J. Giant cell arteritis: visual loss is our major concern. J. Rheumatol. 43, 1458–1461 (2016).
Cid, M. C. et al. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis. Arthritis Rheum. 41, 26–32 (1998).
Haugeberg, G., Paulsen, P. Q. & Bie, R. B. Temporal arteritis in Vest Agder County in southern Norway: incidence and clinical findings. J. Rheumatol. 27, 2624–2627 (2000).
Salvarani, C., Cantini, F. & Hunder, G. G. Polymyalgia rheumatica and giant-cell arteritis. Lancet 372, 234–245 (2008).
Salvarani, C., Cantini, F., Boiardi, L & Hunder, G. GPolymyalgia rheumatica and giant-cell arteritis. N. Engl. J. Med. 347, 261–271 (2002).
Jennings, G. H. Arteritis of the temporal vessels. Lancet 231, 424–428 (1938).
Birkhead, N. C., Wagener, H. P. & Shick, R. M. Treatment of temporal arteritis with adrenal corticosteroids; results in fifty-five cases in which lesion was proved at biopsy. J. Am. Med. Assoc. 163, 821–827 (1957).
Bruce, G. M. Temporal arteritis as a cause of blindness; review of literature and report of a case. Trans. Am. Ophthalmol. Soc. 47, 300–316 (1949).
Cooke, W. T., Cloake, P. C., Govan, A. D. & Colbeck, J. C. Temporal arteritis; a generalized vascular disease. Q. J. Med. 15, 47–75 (1946).
Aiello, P. D., Trautmann, J. C., McPhee, T. J., Kunselman, A. R. & Hunder, G. G. Visual prognosis in giant cell arteritis. Ophthalmology 100, 550–555 (1993).
Liu, G. T., Glaser, J. S., Schatz, N. J. & Smith, J. L. Visual morbidity in giant cell arteritis. Clinical characteristics and prognosis for vision. Ophthalmology 101, 1779–1785 (1994).
Hayreh, S. S., Podhajsky, P. A. & Zimmerman, B. Ocular manifestations of giant cell arteritis. Am. J. Ophthalmol. 125, 509–520 (1998).
Nesher, G. et al. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum. 50, 1332–1337 (2004).
Machado, E. B. et al. Trends in incidence and clinical presentation of temporal arteritis in Olmsted County, Minnesota, 1950–1985. Arthritis Rheum. 31, 745–749 (1988).
Salvarani, C. et al. Risk factors for severe cranial ischaemic events in an Italian population-based cohort of patients with giant cell arteritis. Rheumatology (Oxford) 48, 250–253 (2009).
Singh, A. G. et al. Visual manifestations in giant cell arteritis: trend over 5 decades in a population-based cohort. J. Rheumatol. 42, 309–315 (2015).
Nesher, G., Rubinow, A. & Sonnenblick, M. Trends in the clinical presentation of temporal arteritis in Israel: reflection of increased physician awareness. Clin. Rheumatol. 15, 483–485 (1996).
Liozon, E. et al. Risk factors for permanent visual loss in biopsy-proven giant cell arteritis: a study of 339 patients. J. Rheumatol. 43, 1393–1399 (2016).
Brack, A., Martinez-Taboada, V., Stanson, A., Goronzy, J. J. & Weyand, C. M. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum. 42, 311–317 (1999).
Gonzalez-Gay, M. A. et al. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: predictors and response to treatment. Arthritis Rheum. 41, 1497–1504 (1998).
Berger, C. T., Wolbers, M., Meyer, P., Daikeler, T. & Hess, C. High incidence of severe ischaemic complications in patients with giant cell arteritis irrespective of platelet count and size, and platelet inhibition. Rheumatology (Oxford) 48, 258–261 (2009).
Gonzalez-Gay, M. A. et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine (Baltimore) 88, 227–235 (2009).
Samson, M. et al. Stroke associated with giant cell arteritis: a population-based study. J. Neurol. Neurosurg. Psychiatry. 86, 216–221 (2015).
Zenone, T. & Puget, M. Characteristics of cerebrovascular accidents at time of diagnosis in a series of 98 patients with giant cell arteritis. Rheumatol. Int. 33, 3017–3023 (2013).
Lariviere, D. et al. Extra- and intracranial cerebral vasculitis in giant cell arteritis. Medicine (Baltimore) 93, e265 (2014).
Tomasson, G. et al. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann. Intern. Med. 160, 73–80 (2014).
Lo Gullo, A. et al. Venous thromboembolism and cerebrovascular events in patients with giant cell arteritis: a population-based retrospective cohort study. PLoS ONE 11, e0149579 (2016).
Hayreh, S. S. in Ischemic Optic Neuropathies 199–226 (Springer-Verlag, 2011).
Biousse, V. & Newman, N. Ischemic optic neuropathies. N. Engl. J. Med. 372, 2428–2436 (2015).
Gonzalez-Gay, M. A. et al. Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore) 79, 283–292 (2000).
Hayreh, S. S., Podhajsky, P. A. & Zimmerman, B. Occult giant cell arteritis: ocular manifestations. Am. J. Ophthalmol. 125, 521–526 (1998).
Solans-Laqué, R. et al. Stroke and multi-infarct dementia as presenting symptoms of giant cell arteritis. Report of 7 cases and review of the literature. Medicine (Baltimore) 87, 335–344 (2008).
Pfadenhauer, K., Esser, M. & Berger, K. Vertebrobasilar ischemia and structural abnormalities of the vertebral arteries in active temporal arteritis and polymyalgia rheumatica — an ultrasonographic case-control study. J. Rheumatol. 32, 2356–2360 (2005).
Wilkinson, I. M. & Russel, R. W. Arteries of head and neck in giant cell arteritis. A pathological study to show the pattern of arterial involvement. Arch. Neurol. 27, 378–391 (1972).
Salvarani, C., Giannini, C., Miller, D. V. & Hunder, G. G. Giant cell arteritis: involvement of intracranial arteries. Arthritis Rheum. 55, 985–989 (2006).
Rucker, J. C., Biousse, V. & Newman, N. J. Ischemic optic neuropathies. Curr. Opin. Neurol. 17, 27–35 (2004).
Liozon, E. et al. Risk factors for visual loss in giant cell (temporal) arteritis: a prospective study of 174 patients. Am. J. Med. 111, 211–217 (2001).
Nesher, G. et al. Risk factors for cranial ischemic complications in giant cell arteritis. Medicine (Baltimore) 83, 114–122 (2004).
Muratore, F. et al. Large vessel giant cell arteritis: a cohort study. Rheumatology (Oxford) 54, 463–470 (2015).
Gonzalez-Gay, M. A. et al. Influence of traditional risk factors of atherosclerosis in the development of severe complications of giant cell arteritis. Medicine (Baltimore) 83, 342–347 (2004).
Saleh, M., Turesson, C., Englund, M., Merkel, P. A. & Mohammad, A. J. Visual complications in patients with biopsy-proven giant cell arteritis: a population-based study. J. Rheumatol. 43, 1559–1565 (2016).
Gonzalez-Gay, M. A. et al. Giant cell arteritis: laboratory tests at the time of diagnosis in a series of 240 patients. Medicine (Baltimore) 84, 277–290 (2005).
Cid, M. C. et al. Tissue and serum angiogenic activity is associated with low prevalence of ischemic complications in patients with giant-cell arteritis. Circulation 106, 1664–1671 (2002).
Hernández-Rodríguez, J. et al. Elevated production of interleukin-6 is associated with a lower incidence of disease-related ischemic events in patients with giant-cell arteritis. Angiogenic activity of interleukin-6 as a potential protective mechanism. Circulation 107, 2428–2434 (2003).
Gonzalez-Gay, M. A., Amoli, M. M., Garcia-Porrua, C. & Ollier, W. E. Genetic markers of disease susceptibility and severity in giant cell arteritis and polymyalgia rheumatica. Semin. Arthritis Rheum. 33, 38–48 (2003).
Carmona, F. D. et al. A large-scale genetic analysis reveals a strong contribution of the HLA class II region to giant cell arteritis susceptibility. Am. J. Hum. Genet. 96, 565–580 (2015).
Salvarani, C. et al. PIA1/A2 polymorphism of the platelet glycoprotein receptor IIIA and the risk of cranial ischemic complications in giant cell arteritis. Arthritis Rheum. 56, 3502–3508 (2007).
Feng, D. et al. Increased platelet aggregability associated with platelet GPIIIa PlA2 polymorphism: the Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 19, 1142–1147 (1999).
Rueda, B. et al. A functional variant of vascular endothelial growth factor is associated with severe ischemic complications in giant cell arteritis. J. Rheumatol. 32, 1737–1741 (2005).
Rodríguez-Rodríguez, L. et al. Influence of CD40 rs1883832 polymorphism in susceptibility to and clinical manifestations of biopsy-proven giant cell arteritis. J. Rheumatol. 37, 2076–2080 (2010).
Gonzalez-Gay, M. A. et al. Interferon-gamma gene microsatellite polymorphisms in patients with biopsy-proven giant cell arteritis and polymyalgia rheumatica. Clin. Exp. Rheumatol. 22 (6 Suppl. 36), S18–S20 (2004).
Kaiser, M., Weyand, C. M., Björnsson, J. & Goronzy, J. J. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 41, 623–633 (1998).
Makkuni, D. et al. Is intimal hyperplasia a marker of neuro-ophthalmic complications of giant cell arteritis? Rheumatology (Oxford) 47, 488–490 (2008).
Chatelain, D. et al. Pathological features of temporal arteritis in patients with giant cell arteritis presenting with permanent visual loss. Ann. Rheum. Dis. 68, 84–88 (2009).
Muratore, F. et al. Correlations between histopathological findings and clinical manifestations in biopsy-proven giant cell arteritis. J. Autoimmun. 69, 94–101 (2016).
Espinosa, G. et al. Antiphospholipid antibodies and thrombophilic factors in giant cell arteritis. Semin. Arthritis. Rheum. 31, 12–20 (2001).
Gonzalez-Gay, M. A., Garcia-Porrua, C., Llorca, J., Gonzalez-Louzao, C. & Rodriguez-Ledo, P. Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin. Arthritis Rheum. 30, 249–256 (2001).
Muratore, F. et al. Histopathologic findings of patients with biopsy-negative giant cell arteritis compared to those without arteritis: a population-based study. Arthritis Care Res. (Hoboken) 68, 865–870 (2016).
Achkar, A. A., Lie, J. T., Hunder, G. G., O'Fallon, W. M. & Gabriel, S. E. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann. Intern. Med. 120, 987–992 (1994).
Narváez, J. et al. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin. Arthritis. Rheum. 37, 13–39 (2007).
Bury, D., Joseph, J. & Dawson, T. P. Does preoperative steroid treatment affect the histology in giant cell (cranial) arteritis? J. Clin. Pathol. 65, 1138–1140 (2012).
Mukhtyar, C. et al. EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 68, 318–323 (2009).
Dasgupta, B. et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford) 49, 1594–1597 (2010).
Bienvenu, B. et al. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev. Med. Interne 37, 154–165 (2016).
Hunder, G. G. et al. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann. Intern. Med. 82, 613–618 (1975).
Patil, P. et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin. Exp. Rheumatol. 33 (2 Suppl. 89), S103–S106 (2015).
Diamantopoulos, A. P., Haugeberg, G., Lindland, A. & Myklebust, G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford) 55, 66–70 (2016).
Chevalet, P. et al. A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: a one year follow-up study of 164 patients. J. Rheumatol. 27, 1484–1491 (2000).
Mazlumzadeh, M. et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 54, 3310–3318 (2006).
Hayreh, S. S. & Zimmerman, B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 110, 1204–1215 (2003).
Cornblath, W. T. & Eggenberger, E. R. Progressive visual loss from giant cell arteritis despite high-dose intravenous methylprednisolone. Ophthalmology 104, 854–858 (1997).
Hayreh, S. S., Zimmerman, B. & Kardon, R. H. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of literature. Acta Ophthalmol. Scand. 80, 355–367 (2002).
Danesh-Meyer, H., Savino, P. J. & Gamble, G. G. Poor prognosis of visual outcome after visual loss from giant cell arteritis. Ophthalmology 112, 1098–1103 (2005).
Salvarani, C. et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum. 53, 293–297 (2005).
Chan, C. C., Paine, M. & O'Day, J. Steroid management in giant cell arteritis. Br. J. Ophthalmol. 85, 1061–1064 (2001).
Kupersmith, M. J. et al. Visual performance in giant cell arteritis (temporal arteritis) after 1 year of therapy. Br. J. Ophthalmol. 83, 796–801 (1999).
Foroozan, R. et al. Recovery of visual function in patients with biopsy-proven giant cell arteritis. Ophthalmology 110, 539–542 (2003).
Hayreh, S. S. & Biousse, V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J. Neuroophthalmol. 32, 278–287 (2012).
Villiger, P. M. et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 387, 1921–1927 (2016).
Stone, J. H. et al. Efficacy and safety of tocilizumab in patients with giant cell arteritis: primary and secondary outcomes from a phase 3, randomized, double-blind, placebo-controlled trial [abstract]. Arthritis Rheumatol. 68 (Suppl. 10), 911 (2016).
Hoffman, G. S. et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 46, 1309–1318 (2002).
Lee, M. S., Smith, S. D., Galor, A. & Hoffman, G. S. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum. 54, 3306–3309 (2006).
Jeong, J. & Barra, L. The use of anti-platelet &/or anticoagulant agents in the prevention of large vessel vasculitis-associated ischemic complications: a meta-analysis. Open J. Rheumatol. Autoimmun. Dis. (OJRA) 4, 114–123 (2014).
Martínez-Taboada, V. M., López-Hoyos, M., Narvaez, J. & Munoz-Cacho, P. Effect of antiplatelet/anticoagulant therapy on severe ischemic complications in patients with giant cell arteritis: a cumulative meta-analysis. Autoimmun. Rev. 13, 788–794 (2014).
Mollan, S. P., Sharrack, N., Burdon, M. A. & Denniston, A. K. Aspirin as adjunctive treatment for giant cell arteritis. Cochrane Database Syst. Rev. 3, CD010453 (2014).
Alsolaimani, R. S. et al. Severe intracranial involvement in giant cell arteritis: 5 cases and literature review. J. Rheumatol. 43, 648–656 (2016).
Hayreh, S. S. Anatomy and physiology of the optic nerve head. Trans. Am. Acad. Ophthalmol. Otolaryngol. 78, 240–254 (1974).
Chen, J. J. et al. Evaluating the incidence of arteritic ischemic optic neuropathy and other causes of vision loss from giant cell arteritis. Ophthalmology 123, 1999–2003 (2016).
Ray, J. G., Mamdani, M. M. & Geerts, W. H. Giant cell arteritis and cardiovascular disease in older adults. Heart 91, 324–328 (2005).
Acknowledgements
This article is dedicated to Sohan Singh Hayreh, ophthalmologist and clinical scientist, who has been one of the pioneers in the field of vascular diseases of the eye and the optic nerve.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Fundoscopy
-
A routine examination (also known as ophthalmoscopy) for looking at the back of the eye (fundus)
- Amaurosis fugax
-
Visual loss in one or both eyes that is transient and painless.
- Diplopia
-
Double vision.
- Cortical blindness
-
Blindness resulting from ischaemia of the visual cortex.
- Stenosis
-
Abnormal narrowing of a blood vessel.
- Vasa vasorum
-
A network of small blood vessels that supply the walls of blood vessels
- Cotton wool spots
-
An abnormal manifestation where fluffy white patches are observed on the retina during fundoscopy examination.
- Jaw claudication
-
Pain in the jaw, particularly when talking or eating
Rights and permissions
About this article
Cite this article
Soriano, A., Muratore, F., Pipitone, N. et al. Visual loss and other cranial ischaemic complications in giant cell arteritis. Nat Rev Rheumatol 13, 476–484 (2017). https://doi.org/10.1038/nrrheum.2017.98
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.98
This article is cited by
-
Giant cell arteritis-related cerebrovascular ischemic events: a French retrospective study of 271 patients, systematic review of the literature and meta-analysis
Arthritis Research & Therapy (2023)
-
Large-vessel vasculitis
Nature Reviews Disease Primers (2022)
-
Vision loss in giant cell arteritis: case-based review
Rheumatology International (2022)
-
Neurologic manifestations of giant cell arteritis
Journal of Neurology (2022)
-
Complex oculomotor nerves palsy and incidental ischemic stroke as atypical presentation of giant cell arteritis
Neurological Sciences (2022)