Key Points
-
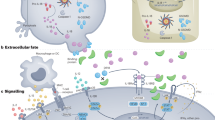
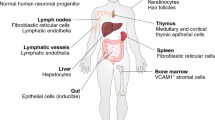
IL-6 is a pleotrophic cytokine with a central role in the integrated immune defence network against infections
-
IL-6 can act via either classic or trans-signalling pathways, which have differential effects on immunocompetence
-
Studies of genetically modified animal models suggest that IL-6 has a role in both the innate and adaptive immune responses that protect the host from a variety of infections
-
Primary immunodeficiency diseases in which IL-6 has been affected either directly or indirectly also provide insights into the role of IL-6 in host defence, especially against bacterial and fungal pathogens
-
Clinical data on IL-6-targeting drugs, largely derived from studies of tocilizumab, suggest that serious and opportunistic infections occur with a frequency similar to that seen with other non-IL-6-targeting biologic agents
-
Neutralizing IL-6 might affect the clinical presentation of serious infections but has minimal effects on response to commonly administered vaccines
Abstract
IL-6 is a pleiotropic cytokine with broad-ranging effects within the integrated immune response. One of the roles of IL-6 is to support immunocompetence, defined as the ability of a host to respond to infections. Understanding the precise role of this cytokine in immunocompetence requires a critical appraisal of data derived from both preclinical and clinical studies. Primary immunodeficiency diseases involving IL-6 or its signalling pathways reveal that IL-6 is critical in the defence against numerous types of pathogens. Studies of IL-6 signalling in preclinical models reveal that selective inhibition of either classic IL-6 signalling or IL-6 trans-signalling has differential effects on the host response to different types of infections. Knowledge of such variation might inform bioengineering of new IL-6-targeting molecules and potentially enable modulation of their toxicity. Clinical studies of IL-6 inhibitors, mainly tocilizumab, reveal that their use is associated with an increased rate of both serious and opportunistic infections generally in the range observed with other non-IL-6 directed biologic therapies. Targeting IL-6 has several other important clinical implications related to diagnosis, management and prevention of infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Calabrese, L. H. & Rose-John, S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol. 10, 720–727 (2014).
Schiff, M. H. et al. Integrated safety in tocilizumab clinical trials. Arthritis Res. Ther. 13, R141 (2011).
Looney, R. J. et al. Guidelines for assessing immunocompetency in clinical trials for autoimmune diseases. Clin. Immunol. 123, 235–243 (2007).
Schaper, F. & Rose-John, S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 26, 475–487 (2015).
Scheller, J. & Rose-John, S. The interleukin 6 pathway and atherosclerosis. Lancet 380, 338 (2012).
Jones, S. A., Scheller, J. & Rose-John, S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 121, 3375–3383 (2011).
Jostock, T. et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 268, 160–167 (2001).
German Clinical Trials Registry. A single-centre, exploratory trial to assess the mechanisms of molecular activity, safety and tolerability of one dose level of FE 999301 by intravenous infusions in patients with active inflammatory bowel disease (IBD). drks-neu.uniklinik-freiburg.de https://drks-neu.uniklinik-freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00010101 (2017).
Moore, P. S., Boshoff, C., Weiss, R. A. & Chang, Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274, 1739–1744 (1996).
Schulte, K. M. & Talat, N. Castleman's disease — a two compartment model of HHV8 infection. Nat. Rev. Clin. Oncol. 7, 533–543 (2010).
Molden, J., Chang, Y., You, Y., Moore, P. S. & Goldsmith, M. A. A. Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272, 19625–19631 (1997).
Mullberg, J. et al. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J. Immunol. 164, 4672–4677 (2000).
Taga, T. & Kishimoto, T. gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 15, 797–819 (1997).
Adam, N. et al. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J. Virol. 83, 5117–5126 (2009).
Chow, D., He, X., Snow, A. L., Rose-John, S. & Garcia, K. C. Structure of an extracellular gp130 cytokine receptor signaling complex. Science 291, 2150–2155 (2001).
Spangler, J. B., Moraga, I., Mendoza, J. L. & Garcia, K. C. Insights into cytokine-receptor interactions from cytokine engineering. Annu. Rev. Immunol. 33, 139–167 (2015).
Suthaus, J. et al. HHV-8-encoded viral IL-6 collaborates with mouse IL-6 in the development of multicentric Castleman disease in mice. Blood 119, 5173–5181 (2012).
Nishimoto, N. et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 106, 2627–2632 (2005).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Harker, J. A., Lewis, G. M., Mack, L. & Zuniga, E. I. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829 (2011).
Zuniga, E. I., Macal, M., Lewis, G. M. & Harker, J. A. Innate and adaptive immune regulation during chronic viral infections. Annu. Rev. Virol. 2, 573–597 (2015).
Hosel, M. et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology 50, 1773–1782 (2009).
Dienz, O. et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 5, 258–266 (2012).
Longhi, M. P. et al. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog. 4, e1000006 (2008).
Smith, K. A. & Maizels, R. M. IL-6 controls susceptibility to helminth infection by impeding Th2 responsiveness and altering the Treg phenotype in vivo. Eur. J. Immunol. 44, 150–161 (2014).
Neveu, W. A. et al. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J. Immunol. 183, 1732–1738 (2009).
Hoge, J. et al. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J. Immunol. 190, 703–711 (2013).
van der Poll, T. et al. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176, 439–444 (1997).
Hochepied, T., Van Molle, W., Bergeri, F. G., Baumann, H. & Libert, C. Involvement of the acute phase protein α1-acid glycoprotein in nonspecific resistance to a lethal gram-negative infection. J. Biol. Chem. 275, 14903–14909 (2000).
Chalaris, A. et al. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood 110, 1748–1755 (2007).
Schumacher, N. et al. Circulating soluble IL-6R but not ADAM17 activation drives mononuclear cell migration in tissue inflammation. J. Immunol. 197, 3705–3715 (2016).
Hurst, S. M. et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14, 705–714 (2001).
Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 (1999).
Gauldie, J., Richards, C., Harnish, D., Lansdorp, P. & Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl Acad. Sci. USA 84, 7251–7255 (1987).
Hochepied, T., Berger, F. G., Baumann, H. & Libert, C. Alpha(1)-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 14, 25–34 (2003).
Simons, J. P. et al. C-reactive protein is essential for innate resistance to pneumococcal infection. Immunology 142, 414–420 (2014).
Mosmann, T. R. & Coffman, R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Bottcher, J. P. et al. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep. 8, 1318–1327 (2014).
Stumhofer, J. S. et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 (2007).
McAleer, J. P. & Kolls, J. K. Mechanisms controlling Th17 cytokine expression and host defense. J. Leukoc. Biol. 90, 263–270 (2011).
Sallusto, F. Heterogeneity of human CD4+ T cells against microbes. Annu. Rev. Immunol. 34, 317–334 (2016).
Rolvering, C. et al. Crosstalk between different family members: IL27 recapitulates IFNgamma responses in HCC cells, but is inhibited by IL6-type cytokines. Biochim. Biophys. Acta 1864, 516–526 (2016).
Vincent, T., Plawecki, M., Goulabchand, R., Guilpain, P. & Eliaou, J. F. Emerging clinical phenotypes associated with anti-cytokine autoantibodies. Autoimmun Rev. 14, 528–535 (2015).
Nanki, T. et al. Suppression of elevations in serum C reactive protein levels by anti-IL-6 autoantibodies in two patients with severe bacterial infections. Ann. Rheum. Dis. 72, 1100–1102 (2013).
Puel, A. et al. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J. Immunol. 180, 647–654 (2008).
Farmand, S. & Sundin, M. Hyper-IgE syndromes: recent advances in pathogenesis, diagnostics and clinical care. Curr. Opin. Hematol. 22, 12–22 (2015).
Vogel, T. P., Milner, J. D. & Cooper, M. A. The ying and yang of STAT3 in human disease. J. Clin. Immunol. 35, 615–623 (2015).
Strand, V. et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res. Ther. 17, 362 (2015).
Schuster, B. et al. Signaling of human ciliary neurotrophic factor (CNTF) revisited. The interleukin-6 receptor can serve as an alpha-receptor for CTNF. J. Biol. Chem. 278, 9528–9535 (2003).
Garbers, C. et al. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer. J. Biol. Chem. 288, 4346–4354 (2013).
Burmester, G. R. et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann. Rheum. Dis. 76, 840–847 (2016).
Genovese, M. C. et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised phase IIb study. Ann. Rheum. Dis. 73, 1607–1615 (2014).
Mease, P. J. et al. The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 68, 2163–2173 (2016).
Smolen, J. S., Weinblatt, M. E., Sheng, S., Zhuang, Y. & Hsu, B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 73, 1616–1625 (2014).
Takeuchi, T. et al. Efficacy and safety of olokizumab in Asian patients with moderate-to-severe rheumatoid arthritis, previously exposed to anti-TNF therapy: results from a randomized phase II trial. Mod. Rheumatol. 26, 15–23 (2016).
Weinblatt, M. E. et al. The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase IIb, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol. 67, 2591–2600 (2015).
Bykerk, V. P. et al. Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann. Rheum. Dis. 71, 1950–1954 (2016).
Iking-Konert, C. et al. ROUTINE-a prospective, multicentre, non-interventional, observational study to evaluate the safety and effectiveness of intravenous tocilizumab for the treatment of active rheumatoid arthritis in daily practice in Germany. Rheumatology (Oxford) 55, 624–635 (2016).
Ishiguro, N. et al. Effectiveness and safety of tocilizumab in achieving clinical and functional remission, and sustaining efficacy in biologics-naive patients with rheumatoid arthritis: the FIRST Bio study. Mod. Rheumatol. 27, 217–226 (2016).
Koike, T. et al. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J. Rheumatol. 41, 15–23 (2014).
Yun, H. et al. Risk of hospitalised infection in rheumatoid arthritis patients receiving biologics following a previous infection while on treatment with anti-TNF therapy. Ann. Rheum. Dis. 74, 1065–1071 (2015).
Xie, F., Yun, H., Bernatsky, S. & Curtis, J. R. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 68, 2612–2617 (2016).
Koike, T. et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann. Rheum. Dis. 70, 2148–2151 (2011).
Yun, H. et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease-modifying therapy. Arthritis Care Res. (Hoboken) 67, 731–736 (2015).
Nagashima, T., Maruyama, A., Kamata, Y. & Minota, S. Unchanged serum viral load and liver function during tocilizumab treatment in a patient with rheumatoid arthritis and hepatitis C virus infection. Rheumatol. Int. 32, 2231–2232 (2012).
Nagashima, T. & Minota, S. Long-term tocilizumab therapy in a patient with rheumatoid arthritis and chronic hepatitis B. Rheumatology (Oxford) 47, 1838–1840 (2008).
Tsuboi, H. et al. A patient with rheumatoid arthritis treated with tocilizumab together with lamivudine prophylaxis after remission of infliximab-reactivated hepatitis B. Mod. Rheumatol. 21, 701–705 (2011).
Nakamura, J. et al. Reactivation of hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int. J. Rheum. Dis. 19, 470–475 (2016).
Freeman, M. L. et al. Cytokines and T-cell homeostasis in HIV infection. J. Infect. Dis. 214 (Suppl. 2), S51–S57 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02049437 (2017).
Wright, H. L., Cross, A. L., Edwards, S. W. & Moots, R. J. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology (Oxford) 53, 1321–1331 (2014).
Gabay, C. et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550 (2013).
Fujiwara, H. et al. Masked early symptoms of pneumonia in patients with rheumatoid arthritis during tocilizumab treatment: a report of two cases. Mod. Rheumatol. 19, 64–68 (2009).
Yanagawa, Y., Hirano, Y., Kato, H. & Iba, T. The absence of typical pneumonia symptoms in a patient with rheumatoid arthritis during tocilizumab and steroid treatment. BMJ Case Rep. 2012, bcr0220125835 (2012).
McMahan, Z. H. & Bingham, C. O. III. Effects of biological and non-biological immunomodulatory therapies on the immunogenicity of vaccines in patients with rheumatic diseases. Arthritis Res. Ther. 16, 506 (2014).
Mori, S. et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann. Rheum. Dis. 72, 1362–1366 (2013).
Tsuru, T. et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod. Rheumatol. 24, 511–516 (2014).
Kapetanovic, M. C. et al. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res. Ther. 16, R2 (2014).
Mori, S. et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann. Rheum. Dis. 71, 2006–2010 (2012).
Bingham, C. O. III et al. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Ann. Rheum. Dis. 74, 818–822 (2015).
Nowell, M. A. et al. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J. Immunol. 171, 3202–3209 (2003).
Nowell, M. A. et al. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J. Immunol. 182, 613–622 (2009).
Richards, P. J. et al. Functional characterization of a soluble gp130 isoform and its therapeutic capacity in an experimental model of inflammatory arthritis. Arthritis Rheum. 54, 1662–1672 (2006).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–538 (2000).
Mitsuyama, K. et al. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut 55, 1263–1269 (2006).
Schuett, H. et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb. Vasc. Biol. 32, 281–290 (2012).
Ullah, M. A. et al. Allergen-induced IL-6 trans-signaling activates gammadelta T cells to promote type 2 and type 17 airway inflammation. J. Allergy Clin. Immunol. 136, 1065–1073 (2015).
Kraakman, M. J. et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 21, 403–416 (2015).
Ruwanpura, S. M. et al. Therapeutic targeting of the IL-6 trans-signalling/mTORC1 axis in pulmonary emphysema. Am. J. Respir. Crit. Care Med. 194, 1494–1505 (2016).
Tecchio, C., Micheletti, A. & Cassatella, M. A. Neutrophil-derived cytokines: facts beyond expression. Front. Immunol. 5, 508 (2014).
Mauer, J. et al. Signalling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15, 423–430 (2014).
Romano, M. et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6, 315–325 (1997).
Klouche, M., Bhakdi, S., Hemmes, M. & Rose-John, S. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J. Immunol. 163, 4583–4589 (1999).
Guerne, P. A., Carson, D. A. & Lotz, M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J. Immunol. 144, 499–505 (1990).
O'Reilly, S., Ciechomska, M., Cant, R. & van Laar, J. M. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-beta (TGF-beta) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J. Biol. Chem. 289, 9952–9960 (2014).
Fielding, C. A. et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40, 40–50 (2014).
Evans, S. S., Repasky, E. A. & Fisher, D. T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat. Rev. Immunol. 15, 335–349 (2015).
Eugster, H. P., Frei, K., Kopf, M., Lassmann, H. & Fontana, A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 28, 2178–2187 (1998).
Bernad, A. et al. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity 1, 725–731 (1994).
Dalrymple, S. A. et al. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 63, 2262–2268 (1995).
Romani, L. et al. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J. Exp. Med. 183, 1345–1355 (1996).
Chai, Z., Gatti, S., Toniatti, C., Poli, V. & Bartfai, T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: a study on IL-6-deficient mice. J. Exp. Med. 183, 311–316 (1996).
Heink, S. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 18, 74–85 (2016).
McFarland-Mancini, M. M. et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J. Immunol. 184, 7219–7228 (2010).
Wunderlich, F. T. et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12, 237–249 (2010).
Yoshida, K. et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc. Natl Acad. Sci. USA 93, 407–411 (1996).
Betz, U. A. et al. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J. Exp. Med. 188, 1955–1965 (1998).
Streetz, K. L. et al. Lack of gp130 expression in hepatocytes promotes liver injury. Gastroenterology 125, 532–543 (2003).
Luchtefeld, M. et al. Signal transducer of inflammation gp130 modulates atherosclerosis in mice and man. J. Exp. Med. 204, 1935–1944 (2007).
Sander, L. E. et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 207, 1453–1464 (2010).
O'Shea, J. J., Gadina, M. & Schreiber, R. D. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109 (Suppl. 1), S121–S131 (2002).
Sakamoto, K. et al. Janus kinase 1 is essential for inflammatory cytokine signaling and mammary gland remodeling. Mol. Cell. Biol. 36, 1673–1690 (2016).
Schwartz, D. M., Bonelli, M., Gadina, M. & O'Shea, J. J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 12, 25–36 (2016).
Lee, C. K. et al. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity 17, 63–72 (2002).
Nguyen-Jackson, H., Panopoulos, A. D., Zhang, H., Li, H. S. & Watowich, S. S. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood 115, 3354–3363 (2010).
Hillmer, E. J., Zhang, H., Li, H. S. & Watowich, S. S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 31, 1–15 (2016).
Kimura, A. et al. SOCS3 is a physiological negative regulator for granulopoiesis and granulocyte colony-stimulating factor receptor signaling. J. Biol. Chem. 279, 6905–6910 (2004).
Peschon, J. J. et al. An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 (1998).
Horiuchi, K. et al. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J. Immunol. 179, 2686–2689 (2007).
Chalaris, A. et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Exp. Med. 207, 1617–1624 (2010).
Kefaloyianni, E. et al. ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 1, e87023 (2016).
Nicolaou, A. et al. Adam17-deficiency promotes atherosclerosis by enhanced TNFR2 signaling in mice. Arterioscler. Thromb. Vasc. Biol. 37, 247–257 (2016).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.H.C. declares that he has acted as a consultant for Bristol–Myers Squib, Genentech-Roche, Jansen, Pfizer, Regeneron, Sanofi-Aventis and UCB, and has acted as a speaker for Bristol–Myers Squib, Genentech, Jansen and UCB. S.R.-J. has acted as a consultant and speaker for AbbVie, Chugai, Genentech Roche, Pfizer and Sanofi. He also declares that he is an inventor on patents owned by CONARIS Research Institute, which develops the sgp130Fc protein olamkicept together with Ferring Pharmaceuticals and he has stock ownership in CONARIS. K.W. declares that he has acted as a consultant to AbbVie, Bristol–Myers Squib, Genentech-Roche, Lilly, Pfizer and UCB, and received a research grant from Bristol–Myers Squib.
Rights and permissions
About this article
Cite this article
Rose-John, S., Winthrop, K. & Calabrese, L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol 13, 399–409 (2017). https://doi.org/10.1038/nrrheum.2017.83
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.83
This article is cited by
-
Association between interleukin-6-174G/C gene polymorphism and asthma severity: exploring the role of total serum IgE, blood eosinophils, and FeNO as markers of type 2 inflammation
Allergy, Asthma & Clinical Immunology (2024)
-
Synergistic inhibition effects of andrographolide and baicalin on coronavirus mechanisms by downregulation of ACE2 protein level
Scientific Reports (2024)
-
Unveiling the molecular Hallmarks of Peyronie’s disease: a comprehensive narrative review
International Journal of Impotence Research (2024)
-
The Human GP130 Cytokine Receptor and Its Expression—an Atlas and Functional Taxonomy of Genetic Variants
Journal of Clinical Immunology (2024)
-
Diagnostic and prognostic role of serum interleukin-6 and carotid ultrasonography to detect subclinical atherosclerosis in patients with RA and ANCA-associated vasculitis
Rheumatology International (2024)