Key Points
-
Haematopoietic stem cell transplantation (HSCT) requires a careful selection of patients according to autoimmune disease, and a consideration of therapeutic alternatives, risks and benefits, and the expertise of the transplantation team
-
The need for graft manipulation before HSCT is uncertain
-
Individualized conditioning regimens might provide increased long-term remission rates, and stem cell rescue could minimize the duration of neutropenia and improve the containment of viruses
-
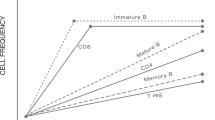
HSCT resets the immune system by renewing the CD4+ T cell compartment, especially within the Treg cell population, and by restoring T cell receptor diversity and function
-
In patients with systemic sclerosis, HSCT results in increased mortality within the first year but a considerable long-term, event-free survival benefit afterwards
Abstract
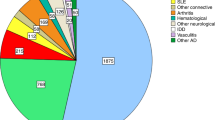
Autologous haematopoietic stem cell transplantation (HSCT) is the only treatment that is able to induce long-term, drug-free and symptom-free remission in several refractory autoimmune rheumatic diseases. Over 3,000 HSCT procedures for rheumatic and nonrheumatic severe autoimmune diseases have been performed worldwide. Specific conditioning regimens are currently used to eradicate the autoreactive immunological memory of patients. Although in vivo immune cell depletion with antithymocyte globulin or anti-CD52 is the norm for many regimens, ex vivo selection of CD34+ stem cells from the graft is controversial. Following the extensive immune depletion associated with serotherapy and chemotherapy, HSCT effectively resets the immune system by renewing the CD4+ T cell compartment, especially the regulatory T cell population. The risk of transplant-related mortality (TRM) within the first 100 days should be weighed against the risk of disease-related mortality, and the careful selection and screening of patients before transplantation is essential. Systemic sclerosis is the first autoimmune disease for which HSCT has been shown, in a randomized, controlled trial, to be associated with increased TRM in the first year but a significant long-term, event-free survival benefit afterwards. In this Review, we discuss the immunological mechanisms of HSCT in various autoimmune diseases and current HSCT regimens. After carefully taking into consideration the risks and benefits of HSCT and alternative therapies, we also discuss the efficacy, complications and proposed indications of this procedure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sureda, A. et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 50, 1037–1056 (2015).
Alexander, T. et al. SCT for severe autoimmune diseases: consensus guidelines of the European Society for Blood and Marrow Transplantation for immune monitoring and biobanking. Bone Marrow Transplant. 50, 173–180 (2015).
Van Bekkum, D. W., Bohre, E. P., Houben, P. F. & Knaan-Shanzer, S. Regression of adjuvant-induced arthritis in rats following bone marrow transplantation. Proc. Natl Acad. Sci. USA 86, 10090–10094 (1989).
Knaan-Shanzer, S., Houben, P., Kinwel-Bohre, E. P. & van Bekkum, D. W. Remission induction of adjuvant arthritis in rats by total body irradiation and autologous bone marrow transplantation. Bone Marrow Transplant. 8, 333–338 (1991).
Baldwin, J. L., Storb, R., Thomas, E. D. & Mannik, M. Bone marrow transplantation in patients with gold-induced marrow aplasia. Arthritis Rheum. 20, 1043–1048 (1977).
Jacobs, P., Vincent, M. & Martell, R. Prolonged remission of severe refractory rheumatoid arthritis following allogeneic bone marrow transplantation for drug-induced aplastic anaemia. Bone Marrow Transplant. 1, 237–239 (1986).
Roubenoff, R., Jones, R. J., Karp, J. E. & Stevens, M. B. Remission of rheumatoid arthritis with the successful treatment of acute myelogenous leukemia with cytosine arabinoside, daunorubicin, and m-AMSA. Arthritis Rheum. 30, 1187–1190 (1987).
Eedy, D. J., Burrows, D., Bridges, J. M. & Jones, F. G. Clearance of severe psoriasis after allogeneic bone marrow transplantation. BMJ 300, 908 (1990).
Yin, J. A. & Jowitt, S. N. Resolution of immune-mediated diseases following allogeneic bone marrow transplantation for leukaemia. Bone Marrow Transplant. 9, 31–33 (1992).
Lowenthal, R. M., Cohen, M. L., Atkinson, K. & Biggs, J. C. Apparent cure of rheumatoid arthritis by bone marrow transplantation. J. Rheumatol. 20, 137–140 (1993).
Marmont, A. M. Immune ablation followed by allogeneic or autologous bone marrow transplantation: a new treatment for severe autoimmune diseases? Stem Cells 12, 125–135 (1994).
Tamm, M., Gratwohl, A., Tichelli, A., Perruchoud, A. P. & Tyndall, A. Autologous haemopoietic stem cell transplantation in a patient with severe pulmonary hypertension complicating connective tissue disease. Ann. Rheum. Dis. 55, 779–780 (1996).
Tyndall, A. & Gratwohl, A. Blood and marrow stem cell transplants in auto-immune disease: a consensus report written on behalf of the European League against Rheumatism (EULAR) and the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 19, 643–645 (1997).
European Society for Blood and Marrow Transplantation. Annual report 2014 [online], (2014).
Snowden, J. A. et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 47, 770–790 (2012).
Van Rhijn-Brouwer, F. C. C. et al. Consensusdocument: Autologe hematopoïetische stamceltransplantatie bij ernstige diffuse cutane systemische sclerose. NT Reum. 19, 17–23 (2016).
Blank, N. et al. Low-dose cyclophosphamide effectively mobilizes peripheral blood stem cells in patients with autoimmune disease. Eur. J. Haematol. 97, 78–82 (2016).
Isidori, A. et al. PBSC mobilization in patients with autoimmune diseases: what's next. Eur. J. Haematol. 97, 5–6 (2015).
DiPersio, J. F. et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for. J. Clin. Oncol. 27, 4767–4773 (2009).
Jagirdar, N. et al. Plerixafor in combination with granulocyte-colony-stimulating factor after chemotherapy increases mobilization efficiency in patients with lymphoma or myeloma: results of a phase II clinical trial. Transfusion 55, 2351–2357 (2015).
Jantunen, E. & Fruehauf, S. Importance of blood graft characteristics in auto-SCT: implications for optimizing mobilization regimens. Bone Marrow Transplant. 46, 627–635 (2011).
Swart, J. F. et al. Changing winds in refractory autoimmune disease in children: clearing the road for tolerance with cellular therapies. Curr. Opin. Rheumatol. 24, 267–273 (2012).
Zand, M. S. et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation 79, 1507–1515 (2005).
Hiepe, F. et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 7, 170–178 (2011).
Alexander, T. et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 113, 214–223 (2009).
Moore, J. et al. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum. 46, 2301–2309 (2002).
Oliveira, M. C. et al. Does ex vivo CD34+ positive selection influence outcome after autologous hematopoietic stem cell transplantation in systemic sclerosis patients? Bone Marrow Transplant. 51, 1–5 (2015).
Alchi, B. et al. Autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: data from the European Group for Blood and Marrow Transplantation registry. Lupus 22, 245–253 (2013).
Admiraal, R. et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2, e194–e203 (2015).
Sensenbrenner, L. L., Steele, A. A. & Santos, G. W. Recovery of hematologic competence without engraftment following attempted bone marrow transplantation for aplastic anemia: report of a case with diffusion chamber studies. Exp. Hematol. 5, 51–58 (1977).
Brodsky, R. A., Sensenbrenner, L. L. & Jones, R. J. Complete remission in severe aplastic anemia after high-dose cyclophosphamide without bone marrow transplantation. Blood 87, 491–494 (1996).
DeZern, A. E. et al. High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases. Medicine (Baltimore) 90, 89–98 (2011).
Dezern, A. E. et al. Repeated treatment with high dose cyclophosphamide for severe autoimmune diseases. Am. J. Blood Res. 3, 84–90 (2013).
Couzin-Frankel, J. Replacing an immune system gone haywire. Science 327, 772–774 (2010).
Muraro, P. A. et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J. Exp. Med. 201, 805–816 (2005).
Dubinsky, A. N., Burt, R. K., Martin, R. & Muraro, P. A. T-cell clones persisting in the circulation after autologous hematopoietic SCT are undetectable in the peripheral CD34+ selected graft. Bone Marrow Transplant. 45, 325–331 (2010).
Wu, Q. et al. Immunological characteristics and T-cell receptor clonal diversity in children with systemic juvenile idiopathic arthritis undergoing T-cell-depleted autologous stem cell transplantation. Immunology 142, 227–236 (2014).
Jones, J. L. et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J. Clin. Invest. 119, 2052–2061 (2009).
Jones, J. L. et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc. Natl Acad. Sci. USA 110, 20200–20205 (2013).
Le Campion, A. et al. Lymphopenia-induced spontaneous T-cell proliferation as a cofactor for autoimmune disease development. Blood 114, 1784–1793 (2009).
Khoruts, A. & Fraser, J. M. A causal link between lymphopenia and autoimmunity. Immunol. Lett. 98, 23–31 (2005).
Krupica, T., Fry, T. J. & Mackall, C. L. Autoimmunity during lymphopenia: a two-hit model. Clin. Immunol. 120, 121–128 (2006).
Schulze-Koops, H. Lymphopenia and autoimmune diseases. Arthritis Res. Ther. 6, 178–180 (2004).
De Kleer, I. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood 107, 1696–1702 (2006).
Farge, D. et al. Analysis of immune reconstitution after autologous bone marrow transplantation in systemic sclerosis. Arthritis Rheum. 52, 1555–1563 (2005).
Delemarre, E. M. et al. Brief report: autologous stem cell transplantation restores immune tolerance in experimental arthritis by renewal and modulation of the teff cell compartment. Arthritis Rheumatol. 66, 350–356 (2014).
Delemarre, E. M. et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood 127, 91–101 (2016).
Darlington, P. J. et al. Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann. Neurol. 73, 341–354 (2013).
Bellutti Enders, F. et al. Correlation of CXCL10, tumor necrosis factor receptor type II, and galectin 9 with disease activity in juvenile dermatomyositis. Arthritis Rheumatol. 66, 2281–2289 (2014).
Enders, F. B. et al. Autologous stem cell transplantation leads to a change in proinflammatory plasma cytokine profile of patients with juvenile dermatomyositis correlating with disease activity. Ann. Rheum. Dis. 74, 315–317 (2014).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Meng, L. et al. Treatment of an autoimmune encephalomyelitis mouse model with nonmyeloablative conditioning and syngeneic bone marrow transplantation. Restor. Neurol. Neurosci. 29, 177–185 (2011).
Herrmann, M. M. Tolerance induction by bone marrow transplantation in a multiple sclerosis model. Blood 106, 1875–1883 (2005).
Burman, J. et al. T-cell responses after haematopoietic stem cell transplantation for aggressive relapsing-remitting multiple sclerosis. Immunology 140, 211–219 (2013).
Abrahamsson, S. V. et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 136, 2888–2903 (2013).
Baraut, J. et al. Peripheral blood regulatory T cells in patients with diffuse systemic sclerosis (SSc) before and after autologous hematopoietic SCT: a pilot study. Bone Marrow Transplant. 49, 349–354 (2014).
Alexander, T. et al. Foxp3+ Helios+ regulatory T cells are expanded in active systemic lupus erythematosus. Ann. Rheum. Dis. 72, 1549–1558 (2013).
Roord, S. T. A. et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood 111, 5233–5241 (2008).
Di Ianni, M. et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011).
Taylor, P. A. L-selectinhi but not the L-selectinlo CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood 104, 3804–3812 (2004).
Steiner, D. et al. Overcoming T cell–mediated rejection of bone marrow allografts by T-regulatory cells: synergism with veto cells and rapamycin. Exp. Hematol. 34, 802–808 (2006).
Trzonkowski, P. et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin. Immunol. 133, 22–26 (2009).
Brunstein, C. G. et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070 (2011).
Schlieer, U., Streitz, M. & Sawitzki, B. Tregs. Curr. Opin. Organ Transplant. 17, 34–41 (2012).
Geissler, E. K. The ONE Study compares cell therapy products in organ transplantation: introduction to a review series on suppressive monocyte-derived cells. Transplant. Res. 1, 11 (2012).
Nguyen, V. H. et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood 111, 945–953 (2008).
Wing, J. B. & Sakaguchi, S. TCR diversity and Treg cells, sometimes more is more. Eur. J. Immunol. 41, 3097–3100 (2011).
Nishio, J. et al. Requirement of full TCR repertoire for regulatory T cells to maintain intestinal homeostasis. Proc. Natl Acad. Sci. USA 112, 12770–12775 (2015).
Muraro, P. A. et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J. Clin. Invest. 124, 1168–1172 (2014).
Brinkman, D. M. C. et al. Autologous stem cell transplantation in children with severe progressive systemic or polyarticular juvenile idiopathic arthritis: Long-term followup of a prospective clinical trial. Arthritis Rheum. 56, 2410–2421 (2007).
Szodoray, P. et al. Immunological reconstitution after autologous stem cell transplantation in patients with refractory systemic autoimmune diseases. Scand. J. Rheumatol. 9742, 110–115 (2011).
Liu, B., Shu, S., Kenny, T. P., Chang, C. & Leung, P. S. C. Stem cell therapy in autoimmune rheumatic diseases: a comprehensive review. Clin. Rev. Allergy Immunol. 47, 244–257 (2014).
Hoy, D. et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 73, 968–974 (2014).
Bathon, J. M. et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N. Engl. J. Med. 343, 1586–1593 (2000).
Weinblatt, M. E. et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 340, 253–259 (1999).
Weinblatt, M. E. et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 48, 35–45 (2003).
Jobanputra, P. et al. A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years. BMJ Open 2, e001395 (2012).
Manders, S. H. M. et al. Cost-effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi-centre randomised trial. Arthritis Res. Ther. 17, 134 (2015).
Hyrich, K. L., Watson, K. D., Lunt, M. & Symmons, D. P. M. Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology 50, 117–123 (2011).
Chang, C. Unmet needs in the treatment of autoimmunity: from aspirin to stem cells. Autoimmun. Rev. 13, 331–346 (2014).
Youssef, J., Novosad, S. A. & Winthrop, K. L. Infection risk and safety of corticosteroid use. Rheum. Dis. Clin. North Am. 42, 157–176 (2016).
Pasquini, M. C. et al. Transplantation for autoimmune diseases in North and South America: a report of the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. 18, 1471–1478 (2012).
Farge, D. et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years' experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica 95, 284–292 (2010).
Gratwohl, A. et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 35, 869–879 (2005).
Van Laar, J. M., Naraghi, K. & Tyndall, A. Haematopoietic stem cell transplantation for poor-prognosis systemic sclerosis. Rheumatology (Oxford) 54, 2126–2133 (2015).
Burt, R. K. et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 378, 498–506 (2011).
Bassler, D. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA 303, 1180 (2010).
Van Laar, J. M. et al. Autologous hematopoietic stem cell transplantation versus intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis. JAMA 311, 2490 (2014).
Hung, E. W. et al. Gastric antral vascular ectasia and its clinical correlates in patients with early diffuse systemic sclerosis in the SCOT trial. J. Rheumatol. 40, 455–460 (2013).
Soysal, T. et al. Bone marrow transplantation for Behçet's disease: a case report and systematic review of the literature. Rheumatology (Oxford) 53, 1136–1141 (2014).
Daikeler, T., Tichelli, A. & Passweg, J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr. Res. 71, 439–444 (2012).
Saccardi, R. et al. Consensus statement concerning cardiotoxicity occurring during haematopoietic stem cell transplantation in the treatment of autoimmune diseases, with special reference to systemic sclerosis and multiple sclerosis. Bone Marrow Transplant. 34, 877–881 (2004).
Daikeler, T. et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood 118, 1693–1698 (2013).
Daikeler, T. et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transplant. 44, 27–33 (2009).
Majhail, N. S. et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 18, 348–371 (2012).
Tichelli, A. et al. Late complications after hematopoietic stem cell transplantation. Expert Rev. Hematol. 2, 583–601 (2009).
Admiraal, R. et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood 128, 2734–2741 (2016).
Bartelink, I. H. et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. 3, e526–e536 (2016).
Gutman, J. A. et al. Chronic graft versus host disease burden and late transplant complications are lower following adult double cord blood versus matched unrelated donor peripheral blood transplantation. Bone Marrow Transplant. 51, 1–6 (2016).
Griffith, L. M. et al. Feasibility of allogeneic hematopoietic stem cell transplantation for autoimmune disease: position statement from a National Institute of Allergy and Infectious Diseases and National Cancer Institute-Sponsored International Workshop, Bethesda, MD, March 1. Biol. Blood Marrow Transplant. 11, 862–870 (2005).
Majhail, N. S. et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 21, 1863–1869 (2015).
Passweg, J. R. et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 50, 476–482 (2015).
Cras, A. et al. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res. Ther. 17, 301 (2015).
Author information
Authors and Affiliations
Contributions
J.F.S. and E.M.D. researched data for article. F.V.W., J.B., J.K., J.V.L. and N.M.W. reviewed and edited the manuscript before submission. J.F.S., E.M.D., J.B. and J.K. wrote the manuscript. All authors contributed substantially to discussion of content.
Corresponding author
Ethics declarations
Competing interests
J.K. is the co-founder and chief scientific officer of Gadeta. The remaining authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Swart, J., Delemarre, E., van Wijk, F. et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol 13, 244–256 (2017). https://doi.org/10.1038/nrrheum.2017.7
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.7
This article is cited by
-
Loss of NLRP6 expression increases the severity of intestinal injury after syngeneic hematopoietic stem cell transplantation
Annals of Hematology (2024)
-
Advances in nanotechnology versus stem cell therapy for the theranostics of multiple sclerosis disease
Applied Nanoscience (2023)
-
Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases
Stem Cell Research & Therapy (2022)
-
Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review
Stem Cell Research & Therapy (2022)
-
Stem cell-based therapy for pulmonary fibrosis
Stem Cell Research & Therapy (2022)