Key Points
-
A fundamental abnormality in rheumatoid arthritis (RA) is the inappropriate growth of immune cells and stromal cells, imposing high metabolic demands to generate energy and biosynthetic precursors
-
In RA, immune cells and stromal cells undergo metabolic adaptations to generate biomass
-
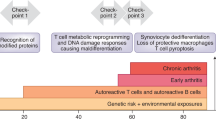
The disease process in RA involves several stages and multiple tissue sites (such as lymphoid organs and joints), each with a distinct metabolic environment
-
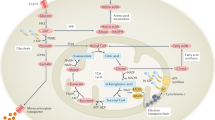
A metabolic signature associated with RA involves the dampening of glycolytic flux and the shunting of glucose into the pentose phosphate pathway in CD4+ T cells
-
In the rheumatoid joint, metabolic intermediates function as signalling molecules and facilitate cell–cell communication, amplifying inflammatory tissue damage
-
The dependence of the rheumatoid disease process on metabolic activity identifies metabolic interference as a potential therapeutic strategy
Abstract
One of the fundamental traits of immune cells in rheumatoid arthritis (RA) is their ability to proliferate, a property shared with the joint-resident cells that form the synovial pannus. The building of biomass imposes high demands for energy and biosynthetic precursors, implicating metabolic control as a basic disease mechanism. During preclinical RA, when autoreactive T cells expand and immunological tolerance is broken, the main sites of disease are the secondary lymphoid tissues. Naive CD4+ T cells from patients with RA have a distinct metabolic signature, characterized by dampened glycolysis, low ATP levels and enhanced shunting of glucose into the pentose phosphate pathway. Equipped with high levels of NADPH and depleted of intracellular reactive oxygen species, such T cells hyperproliferate and acquire proinflammatory effector functions. During clinical RA, immune cells coexist with stromal cells in the acidic milieu of the inflamed joint. This microenvironment is rich in metabolic intermediates that are released into the extracellular space to shape cell–cell communication and the functional activity of tissue-resident cells. Increasing awareness of how metabolites regulate signalling pathways, guide post-translational modifications and condition the tissue microenvironment will help to connect environmental factors with the pathogenic behaviour of T cells in RA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rantapää-Dahlqvist, S. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48, 2741–2749 (2003).
Majka, D. S. & Holers, V. M. Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 48, 2701–2705 (2003).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Kimpimaki, T. & Knip, M. Disease-associated autoantibodies as predictive markers of type 1 diabetes mellitus in siblings of affected children. J. Pediatr. Endocrinol. Metab. 14 (Suppl. 1), 575–587 (2001).
Knip, M. et al. Prediction of type 1 diabetes in the general population. Diabetes Care 33, 1206–1212 (2010).
Gerlag, D. M., Norris, J. M. & Tak, P. P. Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxford) 55, 607–614 (2016).
Law, S. C., Benham, H., Reid, H. H., Rossjohn, J. & Thomas, R. Identification of self-antigen-specific T cells reflecting loss of tolerance in autoimmune disease underpins preventative immunotherapeutic strategies in rheumatoid arthritis. Rheum. Dis. Clin. North Am. 40, 735–752 (2014).
Conigliaro, P. et al. Autoantibodies in inflammatory arthritis. Autoimmun. Rev. 15, 673–683 (2016).
Koppejan, H. et al. Role of anti-carbamylated protein antibodies compared to anti-citrullinated protein antibodies in indigenous North Americans with rheumatoid arthritis, their first-degree relatives, and healthy controls. Arthritis Rheumatol. 68, 2090–2098 (2016).
Dekkers, J., Toes, R. E., Huizinga, T. W. & van der Woude, D. The role of anticitrullinated protein antibodies in the early stages of rheumatoid arthritis. Curr. Opin. Rheumatol. 28, 275–281 (2016).
Imboden, J. B. The immunopathogenesis of rheumatoid arthritis. Annu. Rev. Pathol. 4, 417–434 (2009).
Grimbacher, B., Warnatz, K., Yong, P. F., Korganow, A. S. & Peter, H. H. The crossroads of autoimmunity and immunodeficiency: lessons from polygenic traits and monogenic defects. J. Allergy Clin. Immunol. 137, 3–17 (2016).
Szekanecz, Z. & Koch, A. E. Mechanisms of disease: angiogenesis in inflammatory diseases. Nat. Clin. Pract. Rheumatol. 3, 635–643 (2007).
Koch, A. E. Angiogenesis as a target in rheumatoid arthritis. Ann. Rheum. Dis. 62 (Suppl. 2), ii60–ii67 (2003).
Williams, M. A. & Bevan, M. J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192 (2007).
Van Leeuwen, E. M., Sprent, J. & Surh, C. D. Generation and maintenance of memory CD4+ T cells. Curr. Opim. Immunol. 21, 167–172 (2009).
Dziurla, R. et al. Effects of hypoxia and/or lack of glucose on cellular energy metabolism and cytokine production in stimulated human CD4+ T lymphocytes. Immunol. Lett. 131, 97–105 (2010).
Tripmacher, R. et al. Human CD4+ T cells maintain specific functions even under conditions of extremely restricted ATP production. Eur. J. Immunol. 38, 1631–1642 (2008).
Maciolek, J. A., Pasternak, J. A. & Wilson, H. L. Metabolism of activated T lymphocytes. Curr. Opin. Immunol. 27, 60–74 (2014).
Wang, R. & Green, D. R. Metabolic reprogramming and metabolic dependency in T cells. Immunol. Rev. 249, 14–26 (2012).
Schuster, S., Boley, D., Moller, P., Stark, H. & Kaleta, C. Mathematical models for explaining the Warburg effect: a review focussed on ATP and biomass production. Biochem. Soc. Trans. 43, 1187–1194 (2015).
Stark, H., Fichtner, M., Konig, R., Lorkowski, S. & Schuster, S. Causes of upregulation of glycolysis in lymphocytes upon stimulation. A comparison with other cell types. Biochimie 118, 185–194 (2015).
Icard, P. & Lincet, H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim. Biophys. Acta 1826, 423–433 (2012).
Madeira, V. M. Overview of mitochondrial bioenergetics. Methods Mol. Biol. 810, 1–6 (2012).
Maldonado, E. N. & Lemasters, J. J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 19, 78–84 (2014).
Jose, C., Bellance, N. & Rossignol, R. Choosing between glycolysis and oxidative phosphorylation: a tumor's dilemma? Biochim. Biophys. Acta 1807, 552–561 (2011).
Yang, Z., Fujii, H., Mohan, S. V., Goronzy, J. J. & Weyand, C. M. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J. Exp. Med. 210, 2119–2134 (2013).
Yang, Z. et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci. Transl Med. 8, 331ra38 (2016).
Clem, B. F. et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol. Cancer. Ther. 12, 1461–1470 (2013).
Akimoto, M. et al. Assessment of peripheral blood CD4+ adenosine triphosphate activity in patients with rheumatoid arthritis. Mod. Rheumatol. 23, 19–27 (2013).
Yang, Z., Goronzy, J. J. & Weyand, C. M. The glycolytic enzyme PFKFB3/phosphofructokinase regulates autophagy. Autophagy 10, 382–383 (2014).
Yang, Z., Goronzy, J. J. & Weyand, C. M. Autophagy in autoimmune disease. J. Mol. Med. (Berl.) 93, 707–717 (2015).
Nagel, A. K. & Ball, L. E. Intracellular protein O-GlcNAc modification integrates nutrient status with transcriptional and metabolic regulation. Adv. Cancer Res. 126, 137–166 (2015).
Hanover, J. A. Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J. 15, 1865–1876 (2001).
Golks, A. & Guerini, D. The O-linked N-acetylglucosamine modification in cellular signalling and the immune system. 'Protein modifications: beyond the usual suspects' review series. EMBO Rep. 9, 748–753 (2008).
Grigorian, A. et al. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem. 286, 40133–40141 (2011).
Swamy, M. et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 17, 712–720 (2016).
Forman, H. J., Fukuto, J. M. & Torres, M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 287, C246–C256 (2004).
Sauer, H., Wartenberg, M. & Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 11, 173–186 (2001).
Mittler, R. ROS are good. Trends Plant Sci. 22, 11–19 (2017).
Paull, T. T. Mechanisms of ATM activation. Annu. Rev. Biochem. 84, 711–738 (2015).
Holmdahl, R., Sareila, O., Olsson, L. M., Backdahl, L. & Wing, K. Ncf1 polymorphism reveals oxidative regulation of autoimmune chronic inflammation. Immunol. Rev. 269, 228–247 (2016).
Yau, A. C. & Holmdahl, R. Rheumatoid arthritis: identifying and characterising polymorphisms using rat models. Dis. Model. Mech. 9, 1111–1123 (2016).
Olofsson, P. & Holmdahl, R. Positional cloning of Ncf1 — a piece in the puzzle of arthritis genetics. Scand. J. Immunol. 58, 155–164 (2003).
Gelderman, K. A. et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J. Clin. Invest. 117, 3020–3028 (2007).
Gelderman, K. A., Hultqvist, M., Holmberg, J., Olofsson, P. & Holmdahl, R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc. Natl Acad. Sci. USA 103, 12831–12836 (2006).
Kelkka, T. et al. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxid. Redox Signal. 21, 2231–2245 (2014).
Kraaij, M. D. et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl Acad. Sci. USA 107, 17686–17691 (2010).
Gelderman, K. A. et al. Rheumatoid arthritis: the role of reactive oxygen species in disease development and therapeutic strategies. Antioxid. Redox Signal. 9, 1541–1567 (2007).
Kienhofer, D., Boeltz, S. & Hoffmann, M. H. Reactive oxygen homeostasis — the balance for preventing autoimmunity. Lupus 25, 943–954 (2016).
Shirwany, N. A. & Zou, M. H. AMPK: a cellular metabolic and redox sensor. A minireview. Front. Biosci. (Landmark Ed.) 19, 447–474 (2014).
Dodson, M., Darley-Usmar, V. & Zhang, J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic. Biol. Med. 63, 207–221 (2013).
Yan, H., Zhou, H. F., Hu, Y. & Pham, C. T. Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation. J. Rheum. Dis. Treat. 1, 5 (2015).
Kang, K. Y. et al. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int. Immunopharmacol. 16, 85–92 (2013).
Thornton, C. C. et al. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: a novel mechanism for vascular protection in chronic systemic inflammation. Ann. Rheum. Dis. 75, 439–448 (2016).
Wellen, K. E. & Thompson, C. B. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol. Cell 40, 323–332 (2010).
Pollizzi, K. N. & Powell, J. D. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 36, 13–20 (2015).
Johnson, M. O., Siska, P. J., Contreras, D. C. & Rathmell, J. C. Nutrients and the microenvironment to feed a T cell army. Semin. Immunol. 28, 505–513 (2016).
Perl, A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat. Rev. Rheumatol. 12, 169–182 (2016).
Delgoffe, G. M. & Powell, J. D. Feeding an army: the metabolism of T cells in activation, anergy, and exhaustion. Mol. Immunol. 68, 492–496 (2015).
Palmer, C. S., Ostrowski, M., Balderson, B., Christian, N. & Crowe, S. M. Glucose metabolism regulates T cell activation, differentiation, and functions. Front. Immunol. 6, 1 (2015).
Fearon, U., Canavan, M., Biniecka, M. & Veale, D. J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 12, 385–397 (2016).
Takemura, S. et al. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 167, 1072–1080 (2001).
Seyler, T. M. et al. BLyS and APRIL in rheumatoid arthritis. J. Clin. Invest. 115, 3083–3092 (2005).
Choy, E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 51 (Suppl. 5), v3–v11 (2012).
Karmakar, S., Kay, J. & Gravallese, E. M. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum. Dis. Clin. North Am. 36, 385–404 (2010).
Chang, S. K., Gu, Z. & Brenner, M. B. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol. Rev. 233, 256–266 (2010).
Yang, Z., Matteson, E. L., Goronzy, J. J. & Weyand, C. M. T-cell metabolism in autoimmune disease. Arthritis Res. Ther. 17, 29 (2015).
Li, G. et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 18, 1518–1524 (2012).
Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428–436 (2013).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Feldmann, M. & Maini, S. R. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol. Rev. 223, 7–19 (2008).
Feldmann, M. & Maini, R. N. Perspectives from masters in rheumatology and autoimmunity: can we get closer to a cure for rheumatoid arthritis? Arthritis Rheumatol. 67, 2283–2291 (2015).
LaGory, E. L. & Giaccia, A. J. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 18, 356–365 (2016).
Palazon, A., Goldrath, A. W., Nizet, V. & Johnson, R. S. HIF transcription factors, inflammation, and immunity. Immunity 41, 518–528 (2014).
Lewis, D. M. et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc. Natl Acad. Sci. USA 113, 9292–9297 (2016).
Hockel, M. & Vaupel, P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl Cancer Inst. 93, 266–276 (2001).
Hollander, A. P., Corke, K. P., Freemont, A. J. & Lewis, C. E. Expression of hypoxia-inducible factor 1α by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 44, 1540–1544 (2001).
Muz, B., Khan, M. N., Kiriakidis, S. & Paleolog, E. M. Hypoxia. The role of hypoxia and HIF-dependent signalling events in rheumatoid arthritis. Arthritis Res. Ther. 11, 201 (2009).
Biniecka, M. et al. Dysregulated bioenergetics: a key regulator of joint inflammation. Ann. Rheum. Dis. 75, 2192–2200 (2016).
Tas, S. W., Maracle, C. X., Balogh, E. & Szekanecz, Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat. Rev. Rheumatol. 12, 111–122 (2016).
Treuhaft, P. S. & McCarty, D. J. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 14, 475–484 (1971).
Thomas, D. P. & Dingle, J. T. In vitro studies of rheumatoid synovium; preliminary metabolic comparison between synovial membrane and villi. Br. J. Exp. Pathol. 36, 195–198 (1955).
Goetzl, E. J. et al. A physiological approach to the assessment of disease activity in rheumatoid arthritis. J. Clin. Invest. 50, 1167–1180 (1971).
Yang, X. Y. et al. Energy metabolism disorder as a contributing factor of rheumatoid arthritis: a comparative proteomic and metabolomic study. PLoS ONE 10, e0132695 (2015).
Garcia-Carbonell, R. et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 68, 1614–1626 (2016).
Fujii, W. et al. Monocarboxylate transporter 4, associated with the acidification of synovial fluid, is a novel therapeutic target for inflammatory arthritis. Arthritis Rheumatol. 67, 2888–2896 (2015).
Zhou, R., Wu, X., Wang, Z., Ge, J. & Chen, F. Interleukin-6 enhances acid-induced apoptosis via upregulating acid-sensing ion channel 1a expression and function in rat articular chondrocytes. Int. Immunopharmacol. 29, 748–760 (2015).
Haas, R. et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 13, e1002202 (2015).
Veras, F. P. et al. Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci. Rep. 5, 15171 (2015).
Colville-Nash, P. R. & Scott, D. L. Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann. Rheum. Dis. 51, 919–925 (1992).
Konisti, S., Kiriakidis, S. & Paleolog, E. M. Hypoxia — a key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat. Rev. Rheumatol. 8, 153–162 (2012).
Kelly, B. & O'Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
Weinberg, F. & Chandel, N. S. Mitochondrial metabolism and cancer. Ann. NY Acad. Sci. 1177, 66–73 (2009).
Kim, S. et al. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PLoS ONE 9, e97501 (2014).
Haas, R. et al. Intermediates of metabolism: from bystanders to signalling molecules. Trends Biochem. Sci. 41, 460–471 (2016).
Peti-Peterdi, J., Kishore, B. K. & Pluznick, J. L. Regulation of vascular and renal function by metabolite receptors. Annu. Rev. Physiol. 78, 391–414 (2016).
Salminen, A., Kaarniranta, K., Hiltunen, M. & Kauppinen, A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell Signal. 26, 1598–1603 (2014).
Mills, E. L. & O'Neill, L. A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 46, 13–21 (2016).
Tretter, L., Patocs, A. & Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta 1857, 1086–1101 (2016).
El Kasmi, K. C. & Stenmark, K. R. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin. Immunol. 27, 267–275 (2015).
Mills, E. & O'Neill, L. A. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 24, 313–320 (2014).
Bonnet, C. S. et al. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann. Rheum. Dis. 74, 242–251 (2015).
Littlewood-Evans, A. et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 213, 1655–1662 (2016).
Rubic, T. et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat. Immunol. 9, 1261–1269 (2008).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Shirai, T. et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 213, 337–354 (2016).
Nefla, M., Holzinger, D., Berenbaum, F. & Jacques, C. The danger from within: alarmins in arthritis. Nat. Rev. Rheumatol. 12, 669–683 (2016).
Lavric, M., Miranda-Garcia, M. A., Holzinger, D., Foell, D. & Wittkowski, H. Alarmins firing arthritis: helpful diagnostic tools and promising therapeutic targets. Joint Bone Spine http://dx.doi.org/10.1016/j.jbspin.2016.06.010 (2016).
Dos Santos Jaques, J. A. et al. Activities of enzymes that hydrolyze adenine nucleotides in lymphocytes from patients with rheumatoid arthritis. Cell Biochem. Funct. 31, 395–399 (2013).
Arandjelovic, S., McKenney, K. R., Leming, S. S. & Mowen, K. A. ATP induces protein arginine deiminase 2-dependent citrullination in mast cells through the P2X7 purinergic receptor. J. Immunol. 189, 4112–4122 (2012).
Caporali, F. et al. Human rheumatoid synoviocytes express functional P2X7 receptors. J. Mol. Med. (Berl.) 86, 937–949 (2008).
Fang, F. et al. Expression of CD39 on activated T cells impairs their survival in older individuals. Cell Rep. 14, 1218–1231 (2016).
Pelka, K., Shibata, T., Miyake, K. & Latz, E. Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol. Rev. 269, 60–75 (2016).
Berthelot, J. M., Le Goff, B., Neel, A., Maugars, Y. & Hamidou, M. NETosis: at the crossroads of rheumatoid arthritis, lupus, and vasculitis. Joint Bone Spine http://dx.doi.org/10.1016/j.jbspin.2016.05.013 (2016).
Dai, Y. & Hu, S. Recent insights into the role of autophagy in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 55, 403–410 (2016).
van der Burgh, R. & Boes, M. Mitochondria in autoinflammation: cause, mediator or bystander? Trends Endocrinol. Metab. 26, 263–271 (2015).
Weinberg, S. E., Sena, L. A. & Chandel, N. S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 42, 406–417 (2015).
Yousri, N. A. et al. Diagnostic and prognostic metabolites identified for joint symptoms in the KORA population. J. Proteome Res. 15, 554–562 (2016).
Kell, D. B. & Oliver, S. G. The metabolome 18 years on: a concept comes of age. Metabolomics 12, 148 (2016).
Jiang, M. et al. Serum metabolic signatures of four types of human arthritis. J. Proteome Res. 12, 3769–3779 (2013).
Madsen, R. K. et al. Diagnostic properties of metabolic perturbations in rheumatoid arthritis. Arthritis Res. Ther. 13, R19 (2011).
Lauridsen, M. B. et al. 1H NMR spectroscopy-based interventional metabolic phenotyping: a cohort study of rheumatoid arthritis patients. J. Proteome Res. 9, 4545–4553 (2010).
Young, S. P. et al. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 65, 2015–2023 (2013).
Zhou, J. et al. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 127, 60–67 (2016).
Zabek, A. et al. Application of 1H NMR-based serum metabolomic studies for monitoring female patients with rheumatoid arthritis. J. Pharm. Biomed. Anal. 117, 544–550 (2016).
Surowiec, I. et al. Metabolomics study of fatigue in patients with rheumatoid arthritis naive to biological treatment. Rheumatol. Int. 36, 703–711 (2016).
Weyand, C. M., Yang, Z. & Goronzy, J. J. T-cell aging in rheumatoid arthritis. Curr. Opin. Rheumatol. 26, 93–100 (2014).
Li, Y. et al. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity 45, 903–916 (2016).
de Castro Fonseca, M., Aguiar, C. J., da Rocha Franco, J. A., Gingold, R. N. & Leite, M. F. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun. Signal. 14, 3 (2016).
Dutta, A., Abmayr, S. M. & Workman, J. L. Diverse activities of histone acylations connect metabolism to chromatin function. Mol. Cell 63, 547–552 (2016).
Tedeschi, S. K. & Costenbader, K. H. Is there a role for diet in the therapy of rheumatoid arthritis? Curr. Rheumatol. Rep. 18, 23 (2016).
Nicolau, J., Lequerre, T., Bacquet, H. & Vittecoq, O. Rheumatoid arthritis, insulin resistance, and diabetes. Joint Bone Spine http://dx.doi.org/10.1016/j.jbspin.2016.09.001 (2016).
Rhoads, J. P., Major, A. S. & Rathmell, J. C. Fine tuning of immune metabolism for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. (in press).
Tsokos, G. C. Metabolic control of arthritis: switch pathways to treat. Sci. Transl Med. 8, 331fs8 (2016).
Tripathi, D. N. et al. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc. Natl Acad. Sci. USA 110, E2950–E2957 (2013).
Alexander, A. et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl Acad. Sci. USA 107, 4153–4158 (2010).
Acknowledgements
The work of the authors was supported by grants from the NIH (R01 AR042527, R01 HL 117913, R01 AI108906 and P01 HL129941 to C.M.W. and R01 AI108891, R01 AG045779, U19 AI057266, and I01 BX001669 to J.J.G.).
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Glycolysis
-
An oxygen-independent metabolic pathway that generates two molecules of pyruvate, ATP and NADH from every one molecule of glucose, supporting the tricarboxylic acid cycle and providing intermediates for the pentose phosphate pathway, glycosylation reactions and the synthesis of biomolecules (including serine, glycine, alanine and acetyl-CoA).
- Tricarboxylic acid (TCA) cycle
-
(Also known as the Krebs cycle) A set of connected pathways in the mitochondrial matrix, which metabolize acetyl-CoA derived from glycolysis or fatty acid oxidation, producing NADH and FADH2 for the electron transport chain and precursors for amino acid and fatty acid synthesis.
- Electron transport chain
-
A series of proteins in the inner mitochondrial membrane that transfer electrons from one to the other in a series of redox reactions, resulting in the movement of protons out of the mitochondrial matrix and in the synthesis of ATP.
- Oxidative phosphorylation
-
A metabolic pathway that produces ATP from the oxidation of acetyl-CoA and the transfer of electrons to the electron transport chain via NADH and FADH2.
- Hexosamine biosynthesis pathway
-
A side branch of glycolysis used to synthesize nucleotide sugars from fructose-6-phosphate and glutamine, such as uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), which functions as a glycosyl donor for the posttranslational modification of biomolecules.
- Pentose phosphate pathway (PPP)
-
An anabolic metabolic pathway parallel to glycolysis that branches out from glycolysis with the conversion of glucose-6-phosphate to ribose 5-phosphate and generates the reducing equivalent NADPH, ribose-5-phosphate (used in the synthesis of nucleotides and nucleic acids) and erythrose-4-phosphosphate (used in the synthesis of amino acids).
- Warburg effect
-
The high utilization of glycolysis by rapidly proliferating cells and the subsequent release of lactate into the extracellular milieu; a phenomenon first desribed by Otto Warburg.
Rights and permissions
About this article
Cite this article
Weyand, C., Goronzy, J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol 13, 291–301 (2017). https://doi.org/10.1038/nrrheum.2017.49
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.49
This article is cited by
-
Prodrug-based nanomedicines for rheumatoid arthritis
Discover Nano (2024)
-
Rheumatoid arthritis—recent advances in pathogenesis and the anti-inflammatory effect of plant-derived COX inhibitors
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Machine learning and molecular subtype analyses provide insights into PANoptosis-associated genes in rheumatoid arthritis
Arthritis Research & Therapy (2023)
-
An in situ dual-anchoring strategy for enhanced immobilization of PD-L1 to treat autoimmune diseases
Nature Communications (2023)
-
Signaling pathways in rheumatoid arthritis: implications for targeted therapy
Signal Transduction and Targeted Therapy (2023)