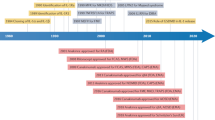
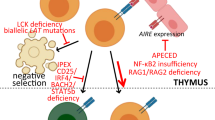
Key Points
-
Immune dysregulation in many primary immunodeficiency syndromes leads to autoimmune disease manifestations
-
Mutations in various genes can lead to immunodeficiencies, as well as to autoimmunity
-
Specific knowledge of these genetic alterations and their pathophysiological consequences will enable the development of new therapeutic approaches
-
Knowledge of primary immunodeficiency syndromes will enable a better understanding of potential infection-related adverse events when DMARDs are used to treat rheumatic diseases
Abstract
Autoimmunity and immunodeficiency were previously considered to be mutually exclusive conditions; however, increased understanding of the complex immune regulatory and signalling mechanisms involved, coupled with the application of genetic analysis, is revealing the complex relationships between primary immunodeficiency syndromes and autoimmune diseases. Single-gene defects can cause rare diseases that predominantly present with autoimmune symptoms. Such genetic defects also predispose individuals to recurrent infections (a hallmark of immunodeficiency) and can cause primary immunodeficiencies, which can also lead to immune dysregulation and autoimmunity. Moreover, risk factors for polygenic rheumatic diseases often exist in the same genes as the mutations that give rise to primary immunodeficiency syndromes. In this Review, various primary immunodeficiency syndromes are presented, along with their pathogenetic mechanisms and relationship to autoimmune diseases, in an effort to increase awareness of immunodeficiencies that occur concurrently with autoimmune diseases and to highlight the need to initiate appropriate genetic tests. The growing knowledge of various genetically determined pathologic mechanisms in patients with immunodeficiencies who have autoimmune symptoms opens up new avenues for personalized molecular therapies that could potentially treat immunodeficiency and autoimmunity at the same time, and that could be further explored in the context of autoimmune rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fischer, A., Provot, J. & Jais, J. Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J. Allergy Clin. Immunol. 140, 1388–1393.e8 (2017).
Okada, Y. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381 (2014).
Grimbacher, B., Warnatz, K., Yong, P. F. K., Korganow, A.-S. & Peter, H.-H. The crossroads of autoimmunity and immunodeficiency: lessons from polygenic traits and monogenic defects. J. Allergy Clin. Immunol. 137, 3–17 (2016).
Carroll, M. C. & Isenman, D. E. Regulation of humoral immunity by complement. Immunity 37, 199–207 (2012).
Ghodke-Puranik, Y. & Niewold, T. B. Immunogenetics of systemic lupus erythematosus: a comprehensive review. J. Autoimmun. 64, 125–136 (2015).
Deng, Y. & Tsao, B. P. Advances in lupus genetics and epigenetics. Curr. Opin. Rheumatol. 26, 1–11 (2014).
Rodero, M. P. & Crow, Y. J. Type I interferon-mediated monogenic autoinflammation: the type I interferono-pathies, a conceptual overview. J. Exp. Med. 213, 2527–2538 (2016).
Rönnblom, L. The importance of the type I interferon system in autoimmunity. Clin. Exp. Rheumatol. 34, 21–24 (2016).
Elkon, K. B. & Stone, V. V. Type I interferon and systemic lupus erythematosus. J. Interferon Cytokine Res. 31, 803–812 (2011).
Lee-Kirsch, M. A. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Lee-Kirsch, M. A., Wolf, C. & Günther, C. Aicardi-Goutières syndrome: a model disease for systemic autoimmunity. Clin. Exp. Immunol. 175, 17–24 (2014).
Molineros, J. E. et al. Admixture mapping in lupus identifies multiple functional variants within IFIH1 associated with apoptosis, inflammation, and autoantibody production. PLoS Genet. 9, e1003222 (2013).
Gao, D. et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA 112, E5699–E5705 (2015).
Notarangelo, L. D., Kim, M.-S., Walter, J. E. & Lee, Y. N. Human RAG mutations: biochemistry and clinical implications. Nat. Rev. Immunol. 16, 234–246 (2016).
Villa, A., Marrella, V., Rucci, F. & Notarangelo, L. D. Genetically determined lymphopenia and autoimmune manifestations. Curr. Opin. Immunol. 20, 318–324 (2008).
Schröder, C. et al. Evaluation of RAG1 mutations in an adult with combined immunodeficiency and progressive multifocal leukoencephalopathy. Clin. Immunol. 179, 1–7 (2016).
Buchbinder, D. et al. Identification of patients with RAG mutations previously diagnosed with common variable immunodeficiency disorders. J. Clin. Immunol. 35, 119–124 (2015).
Henderson, L. A. et al. Expanding the spectrum of recombination-activating gene 1 deficiency: a family with early-onset autoimmunity. J. Allergy Clin. Immunol. 132, 969–971.e2 (2013).
Lee, P. P. et al. The many faces of Artemis-deficient combined immunodeficiency — two patients with DCLRE1C mutations and a systematic literature review of genotype-phenotype correlation. Clin. Immunol. 149, 464–474 (2013).
Walter, J. E. et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest. 125, 4135–4148 (2015).
Atschekzei, F., Ahmad, F., Witte, T., Jacobs, R. & Schmidt, R. E. Limitation of simultaneous analysis of T-cell receptor and κ-deleting recombination excision circles based on multiplex real-time polymerase chain reaction in common variable immunodeficiency patients. Int. Arch. Allergy Immunol. 171, 136–140 (2016).
Gennery, A. R. et al. Antibody deficiency and autoimmunity in 22q11.2 deletion syndrome. Arch. Dis. Child. 86, 422–425 (2002).
Tison, B. E. et al. Autoimmunity in a cohort of 130 pediatric patients with partial DiGeorge syndrome. J. Allergy Clin. Immunol. 128, 1115–1117.e3 (2011).
Hinterberger, M. et al. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat. Immunol. 11, 512–519 (2010).
Cavadini, P. et al. AIRE deficiency in thymus of 2 patients with Omenn syndrome. J. Clin. Invest. 115, 728–732 (2005).
De Martino, L. et al. APECED: a paradigm of complex interactions between genetic background and susceptibility factors. Front. Immunol. 4, 331 (2013).
Ferre, E. M. N. et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight 1, e88782 (2016).
Davies, E. G. et al. Thymus transplantation for complete DiGeorge syndrome: European experience. J. Allergy Clin. Immunol. http://dx.doi.org/10.1016/j.jaci.2017.03.020 (2017).
Bennett, C. L. et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27, 20–21 (2001).
Brunkow, M. E. et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder. Nat. Genet. 27, 68–73 (2001).
Barzaghi, F. et al. Demethylation analysis of the FOXP3 locus shows quantitative defects of regulatory T cells in IPEX-like syndrome. J. Autoimmun. 38, 49–58 (2012).
Barron, L. et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. 185, 6426–6430 (2010).
Von Spee-Mayer, C. et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 75, 1407–1415 (2016).
Friedline, R. H. et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 206, 421–434 (2009).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–276 (2008).
Schubert, D. et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 20, 1410–1416 (2014).
Kuehn, H. S. et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 345, 1623–1627 (2014).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Lo, B. et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 349, 436–440 (2015).
Lopez-Herrera, G. et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am. J. Hum. Genet. 90, 986–1001 (2012).
Stannard, J. N. & Kahlenberg, J. M. Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus. Curr. Opin. Rheumatol. 28, 453–459 (2016).
Gathmann, B. et al. Clinical picture and treatment of 2,212 patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 134, 116–126.e11 (2014).
Lucas, C. L. et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J. Exp. Med. 211, 2537–2547 (2014).
Elkaim, E. et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: a cohort study. J. Allergy Clin. Immunol. 138, 210–218.e9 (2016).
Rao, V. et al. Effective 'activated PI3K δ syndrome'-targeted therapy with the PI3Kδ inhibitor leniolisib. Blood 130, 2307–2316 (2017).
Jackson, C. E. et al. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am. J. Hum. Genet. 64, 1002–1014 (1999).
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009).
Biggs, C. M., Keles, S. & Chatila, T. A. DOCK8 deficiency: Insights into pathophysiology, clinical features and management. Clin. Immunol. 181, 75–82 (2017).
Bacchelli, C. et al. Mutations in linker for activation of T cells (LAT) lead to a novel form of severe combined immunodeficiency. J. Allergy Clin. Immunol. 139, 634–642.e5 (2017).
Keller, B. et al. Early onset combined immunodeficiency and autoimmunity in patients with loss-of-function mutation in LAT. J. Exp. Med. 213, 1185–1199 (2016).
Wilks, A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc. Natl Acad. Sci. USA 86, 1603–1607 (1989).
Darnell, J. J., Kerr, M. & Stark, G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1422 (1994).
Guschin, D. et al. A major role for the protein tyrosin kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 14, 1421–1429 (1995).
Russell, S. M. et al. Interaction of IL-2Rß and gamma c chains with Jak1 and Jak3: Implications for XSCID and XCID. Science 266, 1042–1045 (1994).
Villarino, A. V., Kanno, Y. & Shea, J. J. O. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol. 18, 374–384 (2017).
Del Bel, K., Ragotte, R., Saferali, A. & Lee, S. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J. Allergy Clin. Immunol. 139, 2016–2020 (2017).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
Scott, L. M. et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med. 356, 459–468 (2007).
Zheng, J. et al. Gain-of-function STAT1 mutations impair STAT3 activity in patients with chronic mucocutaneous candidiasis (CMC). Eur. J. Immunol. 45, 2834–2846 (2015).
Liu, L. et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208, 1635–1648 (2011).
Uzel, G. et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J. Allergy Clin. Immunol. 131, 1611–1623 (2013).
van de Veerdonk, F. L. et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 365, 54–61 (2011).
Kong, X.-F. et al. A novel form of human STAT1 deficiency impairing early but not late responses to interferons. Blood 116, 5895–5906 (2010).
Dupuis, S. et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293, 300–303 (2001).
Koskela, H. et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N. Engl. J. Med. 366, 1905–1913 (2012).
Milner, J. D. et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 125, 591–599 (2015).
Buckley, R., Wray, B. & Belmaker, E. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics 49, 59–70 (1972).
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Winthrop, K. L. et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 66, 2675–2684 (2014).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
Hartmann, G. Nucleic acid immunity. Adv. Immunol. 133, 121–169 (2017).
Picard, C. & Belot, A. Does type-I interferon drive systemic autoimmunity? Autoimmun. Rev. 16, 897–902 (2017).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Crow, Y. J. & Casanova, J.-L. STING-associated vasculopathy with onset in infancy — a new interferonopathy. N. Engl. J. Med. 371, 568–571 (2014).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Briggs, T. A. et al. Spondyloenchondrodysplasia due to mutations in ACP5: a comprehensive survey. J. Clin. Immunol. 36, 220–234 (2016).
Atkinson, J. Complement deficiency. Predisposing factor to autoimmune syndromes. Am. J. Med. 85, 45–47 (1988).
Clancy, R. M. et al. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J. Immunol. 184, 2148–2155 (2010).
Savarese, E. et al. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood 107, 3229–3234 (2006).
Yung, S. & Chan, T. M. Anti-DNA antibodies in the pathogenesis of lupus nephritis — the emerging mechanisms. Autoimmun. Rev. 7, 317–321 (2008).
Werwitzke, S. et al. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB × NZW)F1 mouse. Arthritis Rheum. 52, 3629–3638 (2005).
Manderson, A. P. Botto, M. & Walport, M. J. The role of complement in the development of systemic lupus erythematosus. Ann. Rev. Immunol. 22, 431–456 (2004).
Litzman, J. et al. Early manifestation and recognition of C2 complement deficiency in the form of pyogenic infection in infancy. J. Paediatr. Child Health 39, 274–277 (2003).
Ram, S., Lewis, L. A. & Rice, P. A. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin. Microbiol. Rev. 23, 740–780 (2010).
Borzy, M., Gewurz, A., Wolff, L., Houghton, D. & Lovrien, E. Inherited C3 deficiency with recurrent infections and glomerulonephritis. Am. J. Dis. Child 142, 79–83 (1988).
Figueroa, J. E. & Densen, P. Infectious diseases associated with complement deficiencies. Clin. Microbiol. Rev. 4, 359–395 (1991).
Witte, T. et al. Defect of a complement receptor 3 epitope in a patient with systemic lupus erythematosus. J. Clin. Invest. 92, 1181–1187 (1993).
Hom, G. et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N. Engl. J. Med. 358, 900–909 (2008).
Nath, S. K. et al. A nonsynonymous functional variant in integrin-αM (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat. Genet. 40, 152–154 (2008).
Takai, T., Ono, M., Hikida, M., Ohmori, H. & Ravetch, J. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature 379, 346–349 (1996).
Willcocks, L. C. et al. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 107, 7881–7885 (2010).
Niederer, H. A. et al. Copy number, linkage disequilibrium and disease association in the FCGR locus. Hum. Mol. Genet. 19, 3282–3294 (2010).
Wu, J. et al. A novel polymorphism of FcγRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J. Clin. Invest. 100, 1059–1070 (1997).
Sondermann, P. The FcγR/IgG interaction as target for the treatment of autoimmune diseases. J. Clin. Immunol. 36, 95–99 (2016).
Van Parijs, L., Ibraghimov, A. & Abbas, A. K. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity 4, 321–328 (1996).
Enari, M., Hug, H. & Nagata, S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature 375, 78–81 (1995).
Shah, S., Wu, E., Rao, V. K. & Tarrant, T. K. Autoimmune lymphoproliferative syndrome: an update and review of the literature. Curr. Allergy Asthma Rep. 14, 10–14 (2014).
Rieux-Laucat, F., Le Deist, F., Hivroz, C. & Roberts, I. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268, 1347–1350 (1995).
Neven, B. et al. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood 118, 4798–4807 (2011).
Fisher, G. H. et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81, 935–946 (1995).
Watanabe-Fukunaga, R., Brannan, C., Copeland, N., Jenkins, N. & Nagata, S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317 (1992).
Bride, K. L. et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood 127, 17–28 (2016).
Roos, D. Chronic granulomatous disease. Br. Med. Bull. 118, 53–66 (2016).
Roos, D. & de Boer, M. Molecular diagnosis of chronic granulomatous disease. Clin. Exp. Immunol. 175, 139–149 (2013).
Acknowledgements
The work of the authors is supported financially by the Deutsches Zentrum für Gesundheitsforschung (DZIF) (grants to R.E.S. and through the Helmholz Society to B.G.) and by the Deutsche Forschungsgemeinschaft (DFG): Clinical Research Group KFO 250 (grants to R.E.S.and T.W.). The work of B.G. is also supported by the Federal Ministry of Education and Research (BMBF) (grants 01E01303 and 01ZX1306F), the DFG (grants SFB1160 and GR1617-8) and the EU (E-rare programme).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schmidt, R., Grimbacher, B. & Witte, T. Autoimmunity and primary immunodeficiency: two sides of the same coin?. Nat Rev Rheumatol 14, 7–18 (2018). https://doi.org/10.1038/nrrheum.2017.198
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.198
This article is cited by
-
Assessment of autoantibodies in paediatric population with primary immunodeficiencies: a pilot study
BMC Immunology (2023)
-
Glomerulonephritis: immunopathogenesis and immunotherapy
Nature Reviews Immunology (2023)
-
JAKs and STATs from a Clinical Perspective: Loss-of-Function Mutations, Gain-of-Function Mutations, and Their Multidimensional Consequences
Journal of Clinical Immunology (2023)
-
Treg-associated monogenic autoimmune disorders and gut microbial dysbiosis
Pediatric Research (2022)
-
Prof. Dr. med. Reinhold Ernst Schmidt (17.12.1951–23.01.2022)
Zeitschrift für Rheumatologie (2022)