Abstract
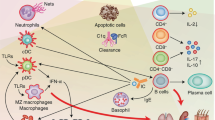
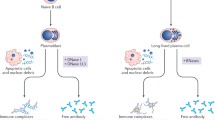
Autoantibodies reactive against host DNA are detectable in the circulation of most people with systemic lupus erythematosus (SLE). The long-held view that antibodies cannot penetrate live cells has been disproved. A subset of lupus autoantibodies penetrate cells, translocate to nuclei, and inhibit DNA repair or directly damages DNA. The result of these effects depends on the microenvironment and genetic traits of the cell. Some DNA-damaging antibodies alone have little impact on normal cells, but in the presence of other conditions, such as pre-existing DNA-repair defects, can become highly toxic. These findings raise new questions about autoimmunity and DNA damage, and reveal opportunities for new targeted therapies against malignancies particularly vulnerable to DNA damage. In this Perspectives article, we review the known associations between SLE, DNA damage and cancer, and propose a theory for the effects of DNA-damaging autoantibodies on SLE pathophysiology and cancer risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fritzler, M. Reflections on Lupus 2013: butterflies, wolves and prophecies. Lupus 22, 1092–1101 (2013).
Lorenz, E. N. Deterministic nonperiodic flow. J. Atmospher. Sci. 20, 130–141 (1963).
Isenberg, D. A., Manson, J. J., Ehrenstein, M. R. & Rahman, A. Fifty years of anti-ds DNA antibodies: are we approaching journey's end? Rheumatology (Oxford) 46, 1052–1056 (2007).
Lisnevskaia, L., Murphy, G. & Isenberg, D. Systemic lupus erythematosus. Lancet 384, 1878–1888 (2014).
Hansen, J. E. et al. Targeting cancer with a lupus autoantibody. Sci. Transl. Med. 4, 157ra142 (2012).
Noble, P. W., Young, M. R., Bernatsky, S., Weisbart, R. H. & Hansen, J. E. A nucleolytic lupus autoantibody is toxic to BRCA2-deficient cancer cells. Sci. Rep. 4, 5958 (2014).
Bernatsky, S. et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J. Autoimmun. 42, 130–135 (2013).
Dey, D., Kenu, E. & Isenberg, D. A. Cancer complicating systemic lupus erythematosus — a dichotomy emerging from a nested case-control study. Lupus 22, 919–927 (2013).
Goobie, G. C., Bernatsky, S., Ramsey-Goldman, R. & Clarke, A. E. Malignancies in systemic lupus erythematosus: a 2015 update. Curr. Opin. Rheumatol. 27, 454–460 (2015).
Ni, J. et al. Lung, liver, prostate, bladder malignancies risk in systemic lupus erythematosus: evidence from a meta-analysis. Lupus 23, 284–292 (2014).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Bashir, S., Harris, G., Denman, M. A., Blake, D. R. & Winyard, P. G. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann. Rheum. Dis. 52, 659–666 (1993).
Benke, P. J. & Belmar, P. Phytohemagglutinin-stimulated lymphocytes from patients with systemic lupus erythematosus demonstrate DNA damage. Metabolism 40, 1037–1042 (1991).
Davies, R. C. et al. Defective DNA double-strand break repair in pediatric systemic lupus erythematosus. Arthritis Rheum. 64, 568–578 (2012).
Harris, G., Asbery, L., Lawley, P. D., Denman, A. M. & Hylton, W. Defective repair of 06-methylguanine in autoimmune diseases. Lancet 2, 952–956 (1982).
Lee, K. J., Dong, X., Wang, J., Takeda, Y. & Dynan, W. S. Identification of human autoantibodies to the DNA ligase IV/XRCC4 complex and mapping of an autoimmune epitope to a potential regulatory region. J. Immunol. 169, 3413–3421 (2002).
Lunec, J., Herbert, K., Blount, S., Griffiths, H. R. & Emery, P. 8-Hydroxydeoxyguanosine. A marker of oxidative DNA damage in systemic lupus erythematosus. FEBS Lett. 348, 131–138 (1994).
McConnell, J. R., Crockard, A. D., Cairns, A. P. & Bell, A. L. Neutrophils from systemic lupus erythematosus patients demonstrate increased nuclear DNA damage. Clin. Exp. Rheumatol. 20, 653–660 (2002).
McCurdy, D., Tai, L. Q., Frias, S. & Wang, Z. Delayed repair of DNA damage by ionizing radiation in cells from patients with juvenile systemic lupus erythematosus and rheumatoid arthritis. Radiat. Res. 147, 48–54 (1997).
Rosenstein, B. S., Rosenstein, R. B. & Zamansky, G. B. Repair of DNA damage induced in systemic lupus erythematosus skin fibroblasts by simulated sunlight. J. Invest. Dermatol. 98, 469–474 (1992).
Lopez-Lopez, L. et al. Mitochondrial DNA damage is associated with damage accrual and disease duration in patients with systemic lupus erythematosus. Lupus 23, 1133–1141 (2014).
Hussain, S. P., Hofseth, L. J. & Harris, C. C. Radical causes of cancer. Nat. Rev. Cancer 3, 276–285 (2003).
Pahan, K. et al. Induction of nitric-oxide synthase and activation of NF-κB by interleukin-12 p40 in microglial cells. J. Biol. Chem. 276, 7899–7905 (2001).
Weckerle, C. E. et al. Large-scale analysis of tumor necrosis factor α levels in systemic lupus erythematosus. Arthritis Rheum. 64, 2947–2952 (2012).
Calmels, S., Hainaut, P. & Ohshima, H. Nitric oxide induces conformational and functional modifications of wild-type p53 tumor suppressor protein. Cancer Res. 57, 3365–3369 (1997).
Cobbs, C. S. et al. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 63, 8670–8673 (2003).
Sidorkina, O., Espey, M. G., Miranda, K. M., Wink, D. A. & Laval, J. Inhibition of poly(ADP-RIBOSE) polymerase (PARP) by nitric oxide and reactive nitrogen oxide species. Free Radic. Biol. Med. 35, 1431–1438 (2003).
Witkiewicz-Kucharczyk, A. & Bal, W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol. Lett. 162, 29–42 (2006).
Yu, C. X., Li, S. & Whorton, A. R. Redox regulation of PTEN by S-nitrosothiols. Mol. Pharmacol. 68, 847–854 (2005).
Okazaki, I. M., Kotani, A. & Honjo, T. Role of AID in tumorigenesis. Adv. Immunol. 94, 245–273 (2007).
Catz, S. D. & Johnson, J. L. Transcriptional regulation of bcl-2 by nuclear factor κB and its significance in prostate cancer. Oncogene 20, 7342–7351 (2001).
Hinz, M. et al. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol. Cell. Biol. 19, 2690–2698 (1999).
da Silva Fonseca, A. M. et al. Polymorphisms in STK17A gene are associated with systemic lupus erythematosus and its clinical manifestations. Gene 527, 435–439 (2013).
Mandel, M., Gurevich, M., Pauzner, R., Kaminski, N. & Achiron, A. Autoimmunity gene expression portrait: specific signature that intersects or differentiates between multiple sclerosis and systemic lupus erythematosus. Clin. Exp. Immunol. 138, 164–170 (2004).
Tsao, B. P. et al. PARP alleles within the linked chromosomal region are associated with systemic lupus erythematosus. J. Clin. Invest. 103, 1135–1140 (1999).
Lin, Y. J. et al. Polymoprhisms in the DNA repair gene XRCC1 and associations with systemic lupus erythematosus risk in the Taiwanese Han Chinese population. Lupus 18, 1246–1251 (2009).
Bassi, C. et al. Efficiency of the DNA repair and polymorphisms of the XRCC1, XRCC3, and XRCC4 DNA repair genes in systemic lupus erythematosus. Lupus 17, 988–995 (2008).
Crow, M. K., Olferiev, M. & Kirou, K. A. Identification of candidate predictors of lupus flare. Trans. Am. Clin. Climatol. Assoc. 126, 184–196 (2015).
Alarcon-Segovia, D., Ruiz-Arguelles, A. & Fishbein, E. Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature 271, 67–69 (1978).
Alarcón-Segovia, D. Antinuclear antibodies: to penetrate or not to penetrate, that was the question. Lupus 10, 315–318 (2001).
Ruiz-Argüelles, A. & Alarcón-Segovia, D. Penetration of autoantibodies into living cells, 2000. Isr. Med. Assoc. J. 3, 121–126 (2001).
Rivadeneyra-Espinoza, L. & Ruiz-Argüelles, A. Cell-penetrating anti-native DNA antibodies trigger apoptosis through both the neglect and programmed pathways. J. Autoimm. 26, 52–56 (2006).
Sun, K. H., Yu, C. L., Tang, S. J. & Sun, G. H. Monoclonal anti-double-stranded DNA autoantibody stimulates the expression and release of IL-1β, IL-6, IL-8, IL-10 and TNF-α from normal human mononuclear cells involving in the lupus pathogenesis. Immunology 99, 352–360 (2000).
Alarcon-Segovia, D., Ruiz-Arguelles, A. & Fishbein, E. Antibody penetration into living cells. I. Intranuclear immunoglobulin in peripheral blood mononuclear cells in mixed connective tissue disease and systemic lupus erythematosus. Clin. Exp. Immunol. 35, 364–375 (1979).
Ehrenstein, M. R. et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 48, 705–711 (1995).
Vlahakos, D. et al. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J. Am. Soc. Nephrol. 2, 1345–1354 (1992).
Galoppin, L. & Saurat, J. H. In vitro study of the binding of antiribonucleoprotein antibodies to the nucleus of isolated living keratinocytes. J. Invest. Dermatol. 76, 264–267 (1981).
Koscec, M. et al. Autoantibodies to ribosomal P proteins penetrate into live hepatocytes and cause cellular dysfunction in culture. J. Immunol. 159, 2033–2041 (1997).
Lisi, S. et al. Fcgamma receptors mediate internalization of anti-Ro and anti-La autoantibodies from Sjogren's syndrome and apoptosis in human salivary gland cell line A-253. J. Oral Pathol. Med. 36, 511–523 (2007).
Hansen, J. E. et al. Intranuclear protein transduction through a nucleoside salvage pathway. J. Biol. Chem. 282, 20790–20793 (2007).
Madaio, M. P. & Yanase, K. Cellular penetration and nuclear localization of anti-DNA antibodies: mechanisms, consequences, implications and applications. J. Autoimmun. 11, 535–538 (1998).
Weisbart, R. H. et al. DNA-dependent targeting of cell nuclei by a lupus autoantibody. Sci. Rep. 5, 12022 (2015).
Yanase, K. et al. A subgroup of murine monoclonal anti-deoxyribonucleic acid antibodies traverse the cytoplasm and enter the nucleus in a time- and temperature-dependent manner. Lab. Invest. 71, 52–60 (1994).
Weisbart, R. H. et al. A conserved anti-DNA antibody idiotype associated with nephritis in murine and human systemic lupus erythematosus. J. Immunol. 144, 2653–2658 (1990).
Spertini, F. et al. Idiotypic vaccination with a murine anti-dsDNA antibody: phase I study in patients with nonactive systemic lupus erythematosus with nephritis. J. Rheumatol. 26, 2602–2608 (1999).
Hansen, J. E. et al. Antibody-mediated p53 protein therapy prevents liver metastasis in vivo. Cancer Res. 67, 1769–1774 (2007).
Hansen, J. E. et al. Antibody-mediated Hsp70 protein therapy. Brain Res. 1088, 187–196 (2006).
Weisbart, R. H. et al. A cell-penetrating bispecific antibody for therapeutic regulation of intracellular targets. Mol. Cancer Ther. 11, 2169–2173 (2012).
Zhan, X. et al. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke 41, 538–543 (2010).
Bohm, I. The apoptosis marker enzyme poly-(ADP-ribose) polymerase (PARP) in systemic lupus erythematosus. Z. Rheumatol. 65, 541–544 (in German) (2006).
Cerboni, B. et al. Poly(ADP-ribose) polymerase activity in systemic lupus erythematosus and systemic sclerosis. Hum. Immunol. 70, 487–491 (2009).
McLornan, D. P., List, A. & Mufti, G. J. Applying synthetic lethality for the selective targeting of cancer. N. Engl. J. Med. 371, 1725–1735 (2014).
Noble, P. W., Chan, G., Young, M. R., Weisbart, R. H. & Hansen, J. E. Optimizing a lupus autoantibody for targeted cancer therapy. Cancer Res. 75, 2285–2291 (2015).
Legostaeva, G. A. et al. Affinity and catalytic heterogeneity of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with multiple sclerosis. J. Cell. Mol. Med. 14, 699–709 (2010).
Nevinsky, G. A. & Buneva, V. N. Peculiarities of abzymes from sera and milk of healthy donors and patients with autoimmune and viral diseases. Biochem. (Mosc.) 74, 945–961 (2009).
Nevinsky, G. A. & Buneva, V. N. Natural catalytic antibodies in norm, autoimmune, viral, and bacterial diseases. ScientificWorldJournal 10, 1203–1233 (2010).
Odintsova, E. S., Dmitrenok, P. S., Timofeeva, A. M., Buneva, V. N. & Nevinsky, G. A. Why specific anti-integrase antibodies from HIV-infected patients can efficiently hydrolyze 21-mer oligopeptide corresponding to antigenic determinant of human myelin basic protein. J. Mol. Recognit. 27, 32–45 (2014).
Bernatsky, S. et al. Decreased breast cancer risk in systemic lupus erythematosus: the search for a genetic basis continues. Lupus 21, 896–899 (2012).
Gadalla, S. M. et al. Breast cancer risk in elderly women with systemic autoimmune rheumatic diseases: a population-based case-control study. Br. J. Cancer 100, 817–821 (2009).
Tessier Cloutier, B. et al. Breast cancer in systemic lupus erythematosus. Oncology 85, 117–121 (2013).
Kohler, B. A. et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl Cancer Inst. 107, djv048 (2015).
De Summa, S. et al. BRCAness: a deeper insight into basal-like breast tumors. Ann. Oncol. 24 (Suppl. 8), viii13–viii21 (2013).
Kaufman, B. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 33, 244–250 (2015).
Wu, H., Goel, V. & Haluska, F. G. PTEN signaling pathways in melanoma. Oncogene 22, 3113–3122 (2003).
Acknowledgements
A.E.C. is the Arthritis Society Chair in Rheumatic Diseases at the University of Calgary, Calgary, Alberta, Canada. D.A.I. is supported by a Biomedical Centre award to University College Hospital and University College London. R.R.-G. is supported by National Institutes of Health (NIH) awards R03CA173822 and P60 AR066464. J.E.H. is supported by the Department of Therapeutic Radiology at Yale School of Medicine and a Yale Center for Clinical Investigation CTSA Scholar Award. This publication was made possible by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), components of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author information
Authors and Affiliations
Contributions
All authors researched data for article, and made substantial contributions to discussions of the content, writing and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Noble, P., Bernatsky, S., Clarke, A. et al. DNA-damaging autoantibodies and cancer: the lupus butterfly theory. Nat Rev Rheumatol 12, 429–434 (2016). https://doi.org/10.1038/nrrheum.2016.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.23
This article is cited by
-
Childhood-onset systemic lupus erythematosus (cSLE) and malignancy: a nationwide multicentre series review
Advances in Rheumatology (2024)
-
The interconnected roles of TRIM21/Ro52 in systemic lupus erythematosus, primary Sjögren’s syndrome, cancers, and cancer metabolism
Cancer Cell International (2023)
-
Age-related mechanisms in the context of rheumatic disease
Nature Reviews Rheumatology (2022)
-
Increased risk of malignancy in patients with systemic lupus erythematosus: population-based cohort study in Korea
Arthritis Research & Therapy (2021)
-
Association of systemic lupus erythematosus autoantibody diversity with breast cancer protection
Arthritis Research & Therapy (2021)