Abstract
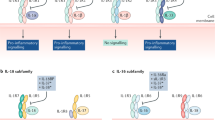
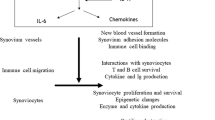
Cytokine-mediated pathways are central to the pathogenesis of rheumatoid arthritis (RA). The purpose of this short Opinion article is to briefly overview the roles of cytokine families in the various phases and tissue compartments of this disease. In particular, we consider the combinatorial role played by cytokines in mediating the overlapping innate and adaptive immune responses associated with disease onset and persistence, and also those cytokine pathways that, in turn, drive the stromal response that is critical for tissue localization and associated articular damage. The success of cytokine inhibition in the clinic is also considerable, not only in offering remarkable therapeutic advances, but also in defining the hierarchical position of distinct cytokines in RA pathogenesis, especially IL-6 and TNF. This hierarchy, in turn, promises to lead to the description of meaningful clinical endotypes and the consequent possibility of therapeutic stratification in future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Firestein, G. S. The disease formerly known as rheumatoid arthritis. Arthritis Res. Ther. 16, 114 (2014).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Zhang, X. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21, 895–905 (2015).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Smolen, J. S. & Aletaha, D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat. Rev. Rheumatol. 11, 276–289 (2015).
Kalliolias, G. D. & Ivashkiv, L. B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2016.169 (2016).
Schett, G. & Manger, B. Interleukin-1 function and role in rheumatic disease. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2016.166 (2016).
Schwartz, D. M., Bonelli, M., Gadina, M. & O'Shea, J. J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2016.167 (2016).
Wicks, I. P. & Roberts, A. W. Targeting GM-CSF in inflammatory diseases. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2016.161 (2016).
Szekanecz, Z. & Koch, A. E. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2016.157 (2016).
Spits, H. & Cupedo, T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30, 647–675 (2012).
Feldmann, M., Brennan, F. M. & Maini, R. N. Rheumatoid arthritis. Cell 85, 307–310 (1996).
Tanaka, T., Narazaki, M. & Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 (2014).
Dennis, G. Jr et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. Ther. 16, R90 (2014).
Garlanda, C., Dinarello, C. A. & Mantovani, A. The interleukin-1 family: back to the future. Immunity 39, 1003–1018 (2013).
Rönnblom, L. & Eloranta, M. L. The interferon signature in autoimmune diseases. Curr. Opin. Rheumatol. 25, 248–253 (2013).
van Holten, J. et al. A multicentre, randomised, double blind, placebo controlled Phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann. Rheum. Dis. 64, 64–69 (2005).
van Nieuwenhuijze, A. et al. GM-CSF as a therapeutic target in inflammatory diseases. Mol. Immunol. 56, 675–682 (2013).
Burmester, G. R. et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann. Rheum. Dis. 72, 1445–1452 (2013).
Lubberts, E. TH17 cytokines and arthritis. Semin. Immunopathol. 32, 43–53 (2010).
Benedetti, G. & Miossec, P. Interleukin 17 contributes to the chronicity of inflammatory diseases such as rheumatoid arthritis. Eur. J. Immunol. 44, 339–347 (2014).
Lubberts, E. The IL-23–IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 11, 415–429 (2015).
McInnes, I. B. et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 74, 694–702 (2015).
Murakami, M. & Hirano, T. A four-step model for the IL-6 amplifier, a regulator of chronic inflammations in tissue-specific MHC class II-associated autoimmune diseases. Front. Immunol. 2, 22 (2011).
Deshpande, P. et al. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. J. Immunol. 190, 1416–1423 (2013).
Di Fusco, D., Izzo, R., Figliuzzi, M. M., Pallone, F. & Monteleone, G. IL-21 as a therapeutic target in inflammatory disorders. Expert Opin. Ther. Targets 18, 1329–1338 (2014).
Kim, H. R., Hwang, K. A., Park, S. H. & Kang, I. IL-7 and IL-15: biology and roles in T-cell immunity in health and disease. Crit. Rev. Immunol. 28, 325–339 (2008).
Baslund, B. et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 52, 2686–2692 (2005).
Vignali, D. A. A. & Kuchroo, V. K. IL-12 family cytokines: immunological playmakers. Nat. Immunol. 13, 722–728 (2012).
Gagliani, N. et al. TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015).
Wang, R.-X. et al. Autoimmune disease. Nat. Med. 20, 633–641 (2014).
van Vollenhoven, R. F., Kinnman, N., Vincent, E., Wax, S. & Bathon, J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a Phase II, randomized, placebo-controlled trial. Arthritis Rheum. 63, 1782–1792 (2011).
Buckley, C. D. Why does chronic inflammation persist: an unexpected role for fibroblasts. Immunol. Lett. 138, 12–14 (2011).
Klein, K., Ospelt, C. & Gay, S. Epigenetic contributions in the development of rheumatoid arthritis. Arthritis Res. Ther. 14, 227 (2012).
Lefevre, S. et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 15, 1414–1420 (2009).
Kapoor, S. R. et al. Metabolic profiling predicts response to anti-tumor necrosis factor α therapy in patients with rheumatoid arthritis. Arthritis Rheum. 65, 1448–1456 (2013).
Young, S. P. et al. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 65, 2015–2023 (2013).
Raza, K. et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 7, R784–R795 (2005).
Filer, A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr. Opin. Pharmacol. 13, 413–419 (2013).
Kiener, H. P. et al. Cadherin 11 promotes invasive behavior of fibroblast-like synoviocytes. Arthritis Rheum. 60, 1305–1310 (2009).
Perlman, H. et al. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J. Immunol. 170, 838–845 (2003).
Vidal, M., Cusick, M. E. & Barabási, A. L. Interactome networks and human disease. Cell 144, 986–998 (2011).
Schett, G. et al. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat. Med. 19, 822–824 (2013).
Acknowledgements
The authors receive funding support from Arthritis Research UK via the Arthritis Research UK Rheumatoid Arthritis Pathogenesis Centre of Excellence.
Author information
Authors and Affiliations
Contributions
All authors researched data for article, made substantial contributions to discussions of the content, wrote the article and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
I.B.M. declares that he has received research funding and honouraria from AbbVie, BMS, MSD, Pfizer, Roche and UCB, all of whom make biologic agents used in the treatment of rheumatoid arthritis. C.D.B. declares that he has received honouraria and research funding from Novartis, Pfizer, Roche and UCB. J.D.I. declares that he has received research funding and honouraria from AbbVie, BMS, Novartis, Janssen, Pfizer and Roche.
PowerPoint slides
Rights and permissions
About this article
Cite this article
McInnes, I., Buckley, C. & Isaacs, J. Cytokines in rheumatoid arthritis — shaping the immunological landscape. Nat Rev Rheumatol 12, 63–68 (2016). https://doi.org/10.1038/nrrheum.2015.171
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.171
This article is cited by
-
Efficacy and pharmacokinetics of ozoralizumab, an anti-TNFα NANOBODY® compound, in patients with rheumatoid arthritis: 52-week results from the OHZORA and NATSUZORA trials
Arthritis Research & Therapy (2023)
-
The PROPER Study: A 48-Week, Pan-European, Real-World Study of Biosimilar SB5 Following Transition from Reference Adalimumab in Patients with Immune‐Mediated Inflammatory Disease
BioDrugs (2023)
-
Differences and similarities in cytokine profiles of macrophage activation syndrome in systemic lupus erythematosus and adult-onset Still’s disease
Clinical and Experimental Medicine (2023)
-
Ozoralizumab: First Approval
Drugs (2023)
-
Efficacy and safety of progressively reducing biologic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis in persistent remission: a study protocol for a non-inferiority randomized, controlled, single-blind trial
Trials (2022)