Key Points
-
Osteoarthritis (OA) is amenable to early prevention and treatment; not all patients with knee OA progress to severe pain or joint replacement, and patients at high risk should be identified
-
Obesity is a major risk factor for OA, and weight loss is effective at reducing the risk of OA, but adherence to interventions is poor and should be addressed by personalized strategies
-
Neuromuscular and proprioceptive training programs are successful in preventing 50% of major knee injuries during sport, which indicates that primary prevention of knee OA is possible
-
Around 50% of individuals sustaining a major knee injury—with or without surgical reconstruction—develop knee OA, and secondary prevention could be valuable in patients with major knee trauma
-
Impaired muscle function—a consequence of physical inactivity—is commonly seen after knee injury, is associated with knee pain, and is an independent risk factor for development of knee OA
-
Biomechanical interventions, such as knee braces and exercise, show promise in altering contact stress and cartilage matrix content, suggesting ways to prevent or delay OA
Abstract
Osteoarthritis (OA) has been thought of as a disease of cartilage that can be effectively treated surgically at severe stages with joint arthroplasty. Today, OA is considered a whole-organ disease that is amenable to prevention and treatment at early stages. OA develops slowly over 10–15 years, interfering with activities of daily living and the ability to work. Many patients tolerate pain, and many health-care providers accept pain and disability as inevitable corollaries of OA and ageing. Too often, health-care providers passively await final 'joint death', necessitating knee and hip replacements. Instead, OA should be viewed as a chronic condition, where prevention and early comprehensive-care models are the accepted norm, as is the case with other chronic diseases. Joint injury, obesity and impaired muscle function are modifiable risk factors amenable to primary and secondary prevention strategies. The strategies that are most appropriate for each patient should be identified, by selecting interventions to correct—or at least attenuate—OA risk factors. We must also choose the interventions that are most likely to be acceptable to patients, to maximize adherence to—and persistence with—the regimes. Now is the time to begin the era of personalized prevention for knee OA.
Similar content being viewed by others
Introduction
Traditionally, osteoarthritis (OA) was diagnosed by the use of radiography, and joint arthroplasty was regarded as the only effective treatment. However, the results of the past 20 years of research have changed our thinking about the disease, and about how and when to treat it. We know today that OA often takes decades to develop and has a range of associated risk factors. We also know that considerable discordance exists between symptoms and structural signs of OA, and current evidence-based clinical guidelines recommend early treatment with education, exercise and weight loss. Between them, hip OA and knee OA are responsible for a major burden of disease, and when they are considered together, they are ranked number 11 of the 291 diseases listed by the WHO according to years lived with disability.1 Although OA was previously regarded as a disease of the elderly, its development starts much earlier than originally thought, and OA is ranked among the top 20 diseases in the 40–45 years age group.2
The current level of knowledge regarding the nature of OA and effective treatments for the disease enable the consideration of preventive strategies and treatments for its first symptoms. This approach is similar to contemporary treatment of cardiovascular disease and diabetes mellitus, and can potentially prevent many years of pain and functional impairment in patients, as well as considerable expenditure on health care. Prevention and early treatment require a reappraisal of the definition and diagnosis of OA. In this Review we outline the classic methods of defining OA, describe a modern framework for its definition, and suggest primary and secondary prevention strategies for three common OA risk factors—joint injury, impaired muscle function and obesity.
What is OA?
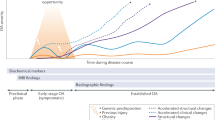
No single definition encompasses all instances of OA. Rather, structural signs of OA are considered to be common outcomes that are arrived at by a number of pathways, involving various risk factors. The discordance between structural signs and symptoms is substantial.3 Symptomatic OA is influenced by comorbidities, pain-processing factors and other personal traits. The current conceptual model describes OA as a whole-person disease (Figure 1) developing along a continuum from early to late stages (Figure 2).
OA is a disease of the whole person. Modifiable and nonmodifiable systemic and mechanical risk factors contribute to the development of joint vulnerability and eventually OA. Comorbidities and pain-related factors affect the development of symptomatic OA. Abbreviation: OA, osteoarthritis.
OA develops slowly, taking 10–15 years to develop from a known initiating trauma. Different techniques can be used to identify individuals at risk of OA and those with early-stage and late-stage disease. For knee OA, primary prevention includes the prevention of knee injury. Secondary prevention includes dietary intervention and exercise in individuals who are overweight, have impaired muscle function or prior joint injury, whereas tertiary prevention involves early treatment of OA to prevent progression of the disease. Abbreviation: OA, osteoarthritis.
Imaging findings of OA
OA affects all the tissues of the synovial joint, which include cartilage, bone, ligament, tendon, synovium and meniscus.4 The relative involvements of each of these tissues can vary from patient to patient, and also throughout the time course of the disease. In most OA research, the disease has been defined by radiography without necessarily considering other structural findings or the symptoms experienced by the patient. This definition does not result in an accurate assessment of the disease, as the presence of radiographic OA is often discordant with the presence of other structural findings and related symptoms.3
A substantial proportion of patients with classic radiographic features of OA do not experience clinically relevant symptoms of the disease. Up to 50% of patients with radiographic knee osteoarthritis (RKOA)—defined by the presence of bony enlargements (osteophytes) and the loss of cartilage (visualized by possible or definite joint-space narrowing of grade ≥2 on the Kellgren and Lawrence scale5)—do not experience regular pain.6 The prevailing (but not unanimous) view is that the development of osteophytes, which are central to the diagnosis of RKOA, is a response to knee injury and instability. In this model, osteophyte formation helps to stabilize the joint by increasing the contact area, thereby reducing instability and pain. Stabilization of joints by osteophyte formation could explain the observation of asymptomatic RKOA. Up to 50% of patients with knee pain suggestive of OA do not have radiographic features of OA.6 The use of highly sensitive MRI, which can detect bone-marrow lesions, synovitis and meniscal abnormalities, can identify abnormalities in a large number of these patients, but the clinical relevance of these lesions is not known. Indeed, in a large study of asymptomatic individuals without radiographic signs of OA, abnormalities thought to be associated with OA were detected in 89% of knees by MRI.7 In a population-based study of ambulatory individuals aged 50–90 years, right-knee meniscal tears were identified by MRI in as few as 19% of women aged 50–59 years and as many as 56% of men aged 70–90 years.8 Among people with RKOA, the prevalence of meniscal tears was 63% in symptomatic individuals and 60% in those who were asymptomatic. Of those with MRI-verified meniscal tears, 61% had not had any pain, aching or stiffness during the preceding month.8 Neither RKOA nor meniscal tears seem to be effective markers of symptomatic knee OA.
Clinical findings of OA
Knee OA can by classified on clinical criteria alone (including pain, age, stiffness, crepitus, bony tenderness and bony enlargement), which make up the inclusion criteria for most clinical trials in this field.9 The use of clinical criteria to diagnose OA is also recommended by the national clinical guidelines in Sweden and Denmark, which explicitly state that radiography is not needed for an OA diagnosis.10,11
Attention should be focused on symptomatic OA (and the phenotypes of pain and function) to identify clinically relevant investigations and treatment strategies capable of reducing the huge burden of the disease. To this end, research is increasingly being directed towards studies of the symptoms of knee OA, usually in combination with structural changes. In parallel, attempts are being made to determine the importance of central and peripheral pain-processing mechanisms. Evidence is emerging of differences in the results of Quantitative Sensory Testing (a noninvasive technique to determine sensory and pain thresholds) and the regulation of genes that are involved in pain processing between subjects with RKOA with and without associated pain.12,13 Although pain and function are often closely related, physicians tend to focus on pain, whereas function and participation restrictions are reported to be equally important to many patients.
Definitions of OA
Although a single definition of OA could help when describing the benefits and cost effectiveness of primary and secondary prevention strategies for knee OA, such a definition might not be possible, or even advisable. A more useful approach could focus on the stages of disease. Commonly, the natural history of OA is described as beginning with normal joint structures, lack of symptoms and normal joint and muscle functions, progressing to total loss of cartilage, which is accompanied by other structural changes, perceived instability and loss of muscle function, with severe pain at night and during activity, impaired physical function and reduced quality of life. This end stage is often called joint failure (Figure 2), in an analogy with heart failure or kidney failure, and is treated by replacement of the joint with an artificial prosthesis.
Completion of the ongoing process of producing classification criteria for early knee OA should aid the performance of clinical studies in early stages of disease, and eventually the definition and assessment of new prevention and treatment strategies. This process could take inspiration from the field of osteoporosis research, in which, after years of debate about different definitions of osteoporosis related to bone density, the bold step was taken to move to a risk-based model for identifying 'at-risk patients' by assessing an individual's 10-year risk of sustaining an osteoporotic fracture. This model would work well in OA as it makes allowances for the heterogeneous nature of the disease, and for the multiple and diverse risk factors that can lead to the development of symptomatic OA (Figure 1). Although models exist to predict the onset of OA, and in particular knee OA, they need to be improved and validated before being used in clinical practice.14,15,16 These models will be useful not only for stratifying risk for prevention strategies, treatment and entry into clinical trials, but also for identification of the risk phenotype of each patient, to enable appropriate interventions. This approach would encompass the key principles of personalized medicine, which seems to be entirely appropriate for diseases such as knee OA, which is responsible for 83% of the burden of disease from OA overall.17 In knee OA, the disease is heterogeneous in terms of risk factors and rates of progression, and no treatment works well for all patients.
Epidemiology of OA
Estimating the size of the problem of knee OA is difficult, because of the multiple definitions of the disease, and the general lack of data from people <50 years old. The largest body of data on knee OA is derived from population-based studies of RKOA. Ideally, we would know the prevalence and incidence of symptomatic knee OA defined by knee pain, alone or associated with clinical signs or radiographic changes, or both. The available data suggest a prevalence of symptomatic RKOA of 20% in individuals >65 years old and an annual incidence rate approaching 1% in women aged 70–89 years.18 In a population-based sample from Sweden the prevalence varied from 8% to 26% in the group aged 56–64 years, depending on whether the definition of disease was based on the combination of pain and RKOA (8% prevalence), clinically defined OA according to ACR criteria (10% prevalence), RKOA alone (17% prevalence) or knee pain alone (26% prevalence).19 Although the prevalence of symptomatic RKOA, clinically defined OA and knee pain were fairly stable in association with age, the prevalence of RKOA alone was nearly 40% in the highest age group (75–84 years).19
Studies of the burden of knee pain in the community have shown that knee pain is predictive of future health-care costs, and OA is associated with considerable costs to individuals and to health-service providers, accounting for >90% of lower-limb joint replacements performed in Western health-care systems.20 OA is also associated with mortality, especially from cardiovascular causes, and physical inactivity is an important mediating factor.21,22,23
Prevention of knee OA
Primary and secondary prevention strategies are necessary to prevent increased rates of OA resulting from an ageing population and increasing rates of obesity and physical inactivity. Strategies that are developed for knee OA might not be transferable to other joints, because of anatomical and other differences.
Primary prevention strategies are intended to prevent the onset of specific diseases via risk reduction, by altering behaviours or exposures that can lead to disease, or by enhancing resistance to the effects of exposure to a disease agent. Preventing knee injury and obesity during adolescence are examples of strategies that are relevant to knee OA. Secondary prevention includes the detection and treatment of risk factors for progression in individuals who are already at risk. Examples relevant to knee OA include the detection and monitoring of weight gain and impairments in proprioceptive acuity, dynamic joint stability and muscle function, and subsequent intervention with weight management and targeted exercise therapy in those who already have sustained a knee injury. OA is a heterogeneous disease with several different phenotypes and a large number of risk factors, which often interact with each other (Figure 1). Three important risk factors, which show promise for both primary and secondary intervention, are obesity, trauma and impaired muscle function.
Epidemiology of obesity
Obesity and related diseases cost the UK economy >UK£3 billion per year, and this cost is expected to rise to £45.5 billion per year by 2050.24 The rates of obesity are increasing dramatically throughout most areas of the developed world as populations become more affluent and sedentary. Most worrying are the high—and also rising—rates of childhood obesity in industrialized countries, which occur before the skeleton is mature.25
Association of obesity with incident knee OA
Obesity is well known to be associated with RKOA, with weaker associations for hand OA and possibly hip OA. Despite initial uncertainty as to whether the obesity was the cause or effect of the OA, prospective cohort studies have demonstrated that obesity precedes the onset of RKOA.26,27,28 Similar associations have been demonstrated for symptomatic RKOA.26,29 Data from the USA demonstrate that the lifetime risk of symptomatic RKOA increases by 30.3% in individuals who are obese compared with those who have normal BMI.30 The adverse effects of obesity are present from an early age; among 1,180 male medical students, each 8 kg increase in weight as a young adult (aged 20–29 years) was associated with a 70% increase in the risk of clinical knee OA >30 years later.28
As well as being overweight or obese, weight gain is associated with the risk of receiving a total knee replacement.31,32 Few data exist on the association of obesity with knee pain. In a cohort of 594 women, BMI at baseline was associated with self-reported knee pain 14 years later.33 Furthermore, the association was independent of RKOA, suggesting the involvement of pain processing rather than structural joint changes.33 In this regard, proinflammatory cytokines originating from adipose tissue can act as pain modulators.34,35 The mechanism of the association between obesity and knee pain is multifactorial, involving mechanical load through the knee joint and levels of physical activity, as well as the systemic inflammatory load caused by cytokine production from adipose tissue. Fat mass, rather than lean mass, might drive the association.36
Weight-reduction strategies
Interventions for weight reduction have been fairly ineffective at the population level, although evidence suggests that a number of successful strategies are available at an individual level. The results of clinical trials have demonstrated the ability of a number of interventions to reduce weight in the short and medium terms, and trials are now addressing the more difficult issue of maintaining weight loss over longer periods of time, as has been reviewed previously.37
Dietary restriction has been shown to reduce weight, but macronutrient (protein, carbohydrate and fat) manipulation within the diet seems to have, at best, a minor effect on weight loss.37 However, for complications of obesity—such as diabetes and heart disease—macronutrient manipulation might well be important.38 Exercise in isolation is less effective than dieting in producing weight loss, particularly in women,39 but exercise is considered pivotal in maintaining weight loss, so diet and exercise are often combined.
Interventions using cognitive behavioural therapy (CBT) can lead to substantial weight loss and do not necessarily need to be intensive.40,41,42,43 The internet and social media are improving the affordability and accessibility of these techniques, so that they can reach a diverse population of overweight people.37
The current gold standard of intervention to achieve weight loss is bariatric surgery. This technique has excellent effectiveness in terms of weight reduction and long-term persistence.44
Although short-term weight loss of 7–10% of body weight is achievable with a number of different strategies, maintaining the loss is difficult. Up to 50% of individuals regain some of their lost weight by 12 months from the initiation of the strategy.37,45 In a diabetes prevention programme, 50% of those involved lost 7% of body weight at 6 months, but only 38% maintained this level of weight loss at a mean of 2.8 years.46 A number of factors are associated with the persistence of weight loss, some of which are irreversible (Box 1). Others are amenable to modification, and should be taken into account when designing weight-loss strategies.
Weight loss and OA
A considerable number of randomized, controlled trials of weight loss have reported OA and joint pain as outcomes, but these trials recruited patients with existing OA and, as such, are tertiary prevention trials, and beyond the scope of this Review, except to state their effectiveness in reducing pain and improving function.47 The effects of weight loss on primary and secondary prevention of knee OA have been investigated in observational studies.
In a study involving 796 women, weight loss of around 5.1 kg reduced the risk of developing symptomatic RKOA over a period of 10 years, relative to women without weight loss (OR 0.46, 95% CI 0.24–0.86).48 In a retrospective study,31 historic weight gain from age 20–50 years was compared in 220 individuals aged 55–75 years with knee arthroplasty for primary OA and 415 individuals from the general population. For knee OA and arthroplasty, weight gain from normal BMI to overweight was associated with higher relative risk than persistent overweight, compared with normal weight over the same age range.31 In the small group of individuals who lost weight, the risk of arthroplasty showed a nonsignificant reduction compared with individuals who maintained normal weight.31 The results of other studies have shown increased risk of knee OA or total knee replacement in association with weight gain, but these studies were underpowered to detect reduced risk with weight loss.32,33
The evidence suggests that obesity leads to knee OA and pain, and that weight loss will reduce both clinical OA and knee pain. Weight loss is achievable with the help of various interventions, and although maintenance of reduced weight is difficult, modifiable predictors have been identified (Box 1). In the current climate of progress towards personalized treatment of disease, interventions for weight loss should be targeted to the individual rather than enforcing homogeneous policies across the whole population.
Epidemiology of knee injury
Early intervention with a focus on the prevention of knee injury in young adults has great potential to reduce the burden of knee OA in the general population. Post-traumatic OA of the hip, knee or ankle is estimated to be responsible for 12% of the overall prevalence of symptomatic OA, with a financial burden of around 0.15% of the total US health-care direct cost outlay.49 The prevention of knee injuries will not only reduce the risk for the individual of life-long consequences in terms of symptoms, function and participation in work and recreational activities, but will also be cost-effective. The cost of implementing prevention programmes is small compared with the potential savings from avoidance of future orthopaedic surgery.
The great majority of knee injuries occur in sport, and sport-related injuries are more frequent in women than in men, which suggests that prevention of injury can be targeted to people at high risk. Although patients are often categorized as having an injury to the anterior cruciate ligament (ACL), these injuries are seldom isolated. On the contrary, concomitant injuries to the menisci, cartilage, bone or other ligaments are nearly always seen.50 The results of a population-based study from Sweden51 showed an incidence rate of 71 physician-diagnosed cruciate ligament injuries per 100,000 persons per year; other soft-tissue injuries were around 10 times as common. The highest incidence of injury was observed during adolescence, but knee injuries continued to occur throughout the adult lifespan.51 The mechanisms of OA development following injury are multifactorial and incompletely understood. These mechanisms seem to involve not only the structures affected by injury, but also inflammatory responses and treatment factors relating to surgery and rehabilitation, as well as personal factors such as obesity and lifestyle, pain processing and genetics.52,53 Prevention strategies should be targeted not only at ACL injuries, but also at overall knee trauma to reduce the future burden from knee OA.
Around 50% of individuals who sustain an ACL injury develop RKOA in 10–15 years, whether or not they have reconstructive surgery.52,54,55,56 Because knee injuries often occur in young adults, these patients subsequently develop OA in their thirties and forties, when they have high demands and expectations of physical activity and participation in work, family and recreational life. As in other OA phenotypes, discrepancies are seen between structural findings and symptoms in those developing OA following a prior injury. Reports of symptoms following knee trauma including ACL injury are mostly restricted to patients who have had surgical reconstruction of the ACL. The relative contributions to the reported symptoms of the injury, the surgical reconstruction, rehabilitation and other surgical treatments are not known. Evidence obtained from a study of 1,197 patients with ACL reconstruction showed that although 66% of patients found their symptom state acceptable at 1–2 years after surgery, 22% were undecided and 12% considered themselves treatment failures.57 Thus, one-third of patients do not deem their symptoms to be acceptable, and similarly, patients with ACL reconstruction report more pain and worse quality of life than population norms.58 ACL injury also affects future physical activity level, with about one-third of those affected not returning to their prior level of sports participation, regardless of whether the injury is treated with surgical reconstruction or rehabilitation alone.59,60,61 Considering the interest and increasing participation rates in soccer, basketball and other sports with a high risk of joint injury, and the involvement of heavier players in many sports, the incidence of OA as a consequence of joint trauma is likely to increase in the future.53
The identification of young people at risk of OA could involve screening for risk factors such as knee injuries requiring medical attention, surgery to the joint, persistent pain within the past month, overweight or obesity, physical inactivity, impaired muscle function and family history of OA. Most epidemiological studies of the incidence of OA have had a lower age limit of 50 for participants, but future studies should include people in their twenties, thirties and forties, to assess those who sustained major knee injuries as adolescents—around half of whom develop RKOA within 10–15 years of the injury.
Primary prevention of knee injury
A meta-analysis including data derived from 27,000 individuals found that neuromuscular and proprioceptive training programs are successful in preventing around 50% of ACL injuries.62 Principles of neuromuscular training for the prevention of knee injury are similar to those used in the treatment of knee injury (Box 2). These programmes typically take 10–20 min to perform, and commonly substitute for the regular warm-up session prior to sports practice 2–3 times weekly. The programmes usually also involve education in the awareness of high-risk positions. This form of prevention seems to be equally effective in all subgroups of individuals analysed.62
As with all behavioural changes, maintaining the new behaviour—in this case, including neuromuscular training in warm-up sessions prior to sports practice—is a challenge. Both the immediate and long-term effects are highly dependent on adherence to the training. In Norway, the use of physical therapists instead of coaches to train female handball players in a neuromuscular injury-prevention programme was associated with adherence during the specified intervention period.63 However, when the intervention period was finished, the injury rate increased and was at least as high as before the intervention started. An information campaign was then developed, targeting team coaches and managers in major cities around the country. The campaign involved an educational DVD and a website describing sport-specific exercise programmes, as well as a range of other activities, performed over a period of >10 years. The incidence of ACL injury in team handball was monitored and found to decrease to levels below those seen during the initial intervention period.63 In sports, many of the choices that determine the risk of injury to a player are made by the coach, so the implementation of injury-prevention programmes by coaches represents a valuable opportunity to effect change.63 The alternative, in which the players themselves have responsibility for preventive training, is ineffective.
Evidence shows that neuromuscular training performed for 15–20 min, 2–3 times per week, prevents 50% of ACL injuries. The current challenge is to increase uptake of such training (which shows great variation across sports and countries) in high-risk groups such as children and adolescent players. In this respect, the inclusion of injury-prevention programmes during physical-education classes in schools could have a substantial public-health effect. Guidance on well-tested interventions, along with additional information, is easily accessible on the internet, to help organizations, politicians, insurance companies, schools and other stakeholders to facilitate uptake.64,65
Secondary prevention of knee OA
Individuals who sustain a knee injury in youth sports are at increased risk of being symptomatic, having impaired physical function, and being overweight or obese 3–10 years later, compared with uninjured controls matched for age, sex and sport.66 These findings suggest that risk factors predictive of OA in the general population are also present at a younger age in those with prior knee injury.
Compared with uninjured individuals, knee injuries are known to increase the risk of OA, and orthopaedic knee surgery is associated with the risk of OA and joint replacement at a younger age.54,67 The group of patients with injury overlaps with the surgery group, but not completely. For example, arthroscopic partial meniscectomy (APM) is the most commonly performed arthroscopic knee procedure, and is most often performed in middle-aged individuals without any prior high-impact trauma.68 Middle-aged patients undergoing APM have a sevenfold increased risk of developing symptomatic RKOA, compared with controls matched for age, sex and BMI.69 Individuals who have had major knee trauma, or orthopaedic surgery, make up easily identifiable target groups in whom secondary prevention could be valuable. Although the causes of OA vary in different patients, the consensus is that OA is a mechanically driven disease, which is evident in individuals who have had injury or surgery—or both—in whom the joint structure is affected, so that the joint is less stable and more vulnerable. As an example, relative to joints without surgery, removal of meniscal tissue increases local contact pressures and the risk of future OA.70
In addition to advice on achieving a healthy lifestyle, including maintaining a healthy body weight and regular physical activity, prevention strategies for those who are at risk of knee OA owing to injury or surgery should focus on biomechanical interventions with the ability to improve joint stability and decrease pain. As with the prevention of injury, neuromuscular exercise therapy can be used for this purpose. Neuromuscular exercise therapy is based on biomechanical principles, targets the sensorimotor system, stabilizes the joint while in motion and improves the patient's trust in their knee (Box 2, Figure 3).71 Neuromuscular exercise therapy, like aerobic exercise and strength training, also provides effective pain relief in individuals with established OA.72,73,74 The first neuromuscular exercise program developed for use in patients with OA was published in 2010.75 Meta-analyses of the effectiveness of neuromuscular exercise should include consideration of additional outcomes such as perceived instability, tendency to give way, confidence in the knee and biomechanical outcomes.
a | Whereas aerobic exercise aims to improve cardiovascular fitness, and strength training aims to increase muscle strength and muscle mass, neuromuscular exercise aims to improve sensorimotor control and obtain functional joint stabilization. The rationale for the use of neuromuscular training is the existence of sensorimotor deficiencies, symptoms of pain, functional instability and functional limitation. b | The targets for improvement are postural control, proprioception, muscle activation, muscle strength and coordination. The exercises involve multiple joints and muscle groups, closed kinetic chains, and lying, sitting and standing positions. Good movement quality with appropriate positioning of the hip, knee and foot in relation to each other is emphasized. The level of training is determined by the patient's sensorimotor control and quality of movement. Training progresses by introducing more-challenging support surfaces, engaging more body parts simultaneously and adding external stimuli, as well as varying the type, speed and direction of movement. Examples of external stimuli include throwing a ball, catching a ball and sudden, unexpected movements.
Exercise therapy is different from physical activity with regard to definition, purpose and goal. Although physical activity is good for general health, and is defined as “any bodily movement produced by skeletal muscles that requires energy expenditure”,76 exercise therapy is good for the joint at risk of OA and is defined as “A regimen or plan of physical activities designed and prescribed for specific therapeutic goals. Its purpose is to restore normal musculoskeletal function or to reduce pain caused by diseases or injuries.”71,77 Neuromuscular exercise therapy is more effective than strength training alone in improving outcomes following ACL injury,78 and in many countries is the main component of ACL rehabilitation.79 However, strength training is also important in knee-injury rehabilitation, because muscle weakness is common following injury and is an independent risk factor for the development of OA.80
Access to rehabilitation differs across countries and health-care systems. In some countries, patients with surgical reconstruction of the knee are not offered any structured exercise therapy at all, but are left to perform more general physical activities on their own. The available knee-ligament registries are focused on surgical patients, so the effect of exercise alone following a knee-ligament injury cannot be determined. The only high-quality, randomized study in young, active adults (comparing individuals treated with rehabilitation and early ACL reconstruction to those treated with rehabilitation alone and optional delayed ACL reconstruction) showed no difference in pain, other symptoms, function, quality of life, return to sport or RKOA at 2 years or 5 years.61,81 The results of a randomized study in 45 middle-aged patients with partial medial meniscectomy who were at high risk of OA suggest that neuromuscular exercise can improve cartilage matrix content.82
Exercise therapy is an active approach involving the sensorimotor system—including the muscles—to improve biomechanics; passive approaches are also available to improve joint biomechanics. Most commonly, knee braces and shoe modifications have been studied, alone or in combination. Typically, valgus braces (which force the knee in a medial direction) and lateral-wedge foot orthotics (which force the foot towards a more pronated position) have been tested in patients with established medial knee OA. This combination, or the brace alone, decreases pain and somewhat shifts the load in the knee from the medial to the lateral side.83,84,85,86,87,88,89 This change in load or contact stress might delay or prevent the onset of OA. In a randomized study in patients with patello-femoral OA, bracing was associated with pain relief and a decreased volume of bone marrow lesions in the affected compartment.90 These strategies could be used to prevent future OA, but poor compliance is currently a problem,88,89 and further development of devices and clinical studies are needed.
Personalizing prevention strategies
Motivation and adherence are key components in successful lifestyle changes. Ultimately, the responsibility for achieving a healthy lifestyle lies with the individual, and surveys show that large parts of the population are willing to make the required changes. However, the role of the clinician in providing motivation and support cannot be overemphasized.
In the clinician's office
Clinicians with positive attitudes and beliefs, who take time to explore the goals and barriers that patients perceive to be important, are more successful in achieving lifestyle changes in their patients than less-engaged clinicians. However, this approach is time-consuming and rarely feasible in a busy practice. Referral to patient education and self-management programmes is, therefore, an attractive option to inform patients about the disease that they are at risk of or have early symptoms of, as well as their future prospects, and the effects, advantages and disadvantages of available treatment options.
Patient education
Patient education is often delivered in groups to enable interaction between participants, but is also available to individuals via the internet. Shared-decision-making tools enable patients, carers and clinicians to participate jointly in the choice of care pathway.91 A choice of educational packages should be provided, to enable patients to have a choice and a personalized education. Checklists of known barriers to change and strategies to overcome them have been developed.92,93 Motivating factors for patients include the presence of social support, opportunities for organized exercise conducted by a professional, the availability of an exercise partner, and familiarity with exercise tasks.94
Personalized exercise and weight loss
Although different types of exercise have similar pain-relieving effects,74,95 exploratory analyses suggest potential benefits to targeting deficits in individual patients with specific exercises.96 A patient suffering from varus thrust during walking (which is related to joint instability and lack of confidence in the knee) is likely to benefit from neuromuscular exercise, whereas an obese patient with muscle weakness is likely to benefit from strength training.96 Strategies for weight loss and exercise, as well as the use of appliances for biomechanical modification, should be tailored to the individual patient, rather than using a 'one size fits all' approach.
Conclusions
Knee OA is a common disease, which is predicted to become more prevalent as longevity and rates of obesity and physical inactivity increase. The current armoury of nonsurgical treatments for knee OA is aimed at providing relief from the symptoms of the disease, and no validated disease-modifying drugs are being marketed. The employment of prevention strategies is, therefore, essential to prevent an epidemic of knee OA.
Not all patients with RKOA experience knee pain, and most of those with RKOA will not progress to require surgical joint replacement. Early identification of individuals who are at risk of developing knee pain and OA is essential, to target prevention strategies more effectively. Neuromuscular exercise programmes are successful in preventing half of the knee injuries that occur during adolescence, suggesting that primary prevention of knee OA is possible. Appropriate prevention strategies, including weight loss and exercise programmes, should be identified for each patient by selecting interventions to correct, or at least attenuate, risk factors for OA. These interventions must also be acceptable to the patients, to maximize adherence to—and persistence with—the regimes. Now is the time to start the era of personalized prevention for knee OA.
References
Cross, M. et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73, 1323–1330 (2014).
Institute for Health Metrics and Evaluation. GBD Data Visualizations [online], (2015).
Javaid, M. K. et al. Individual magnetic resonance imaging and radiographic features of knee osteoarthritis in subjects with unilateral knee pain: the health, aging, and body composition study. Arthritis Rheum. 64, 3246–3255 (2012).
Lane, N. E. et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 19, 478–482 (2011).
Kellgren, J. H. & Lawrence, J. S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16, 494–502 (1957).
Lawrence, J. S., Bremner, J. M. & Bier, F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann. Rheum. Dis. 25, 1–24 (1966).
Guermazi, A. et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ 345, e5339 (2012).
Englund, M. et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N. Engl. J. Med. 359, 1108–1115 (2008).
Altman, R. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 29, 1039–1049 (1986).
Socialstyrelsen (SoS). Nationella riktlinjer för rörelseorganens sjukdomar 2012: Osteoporos, artros, inflammatoriskryggsjukdom och ankyloserande spondylit, psoriasisartrit och reumatoid artrit: Stöd för styrning och ledning [Swedish] (SoS, 2012).
Sundhedsstyrelsen. Knæartrose–nationale kliniske retningslinjer og faglige visitationsretningslinjer [online], (2012).
Soni, A. et al. Neuropathic features of joint pain: a community-based study. Arthritis Rheum. 65, 1942–1949 (2013).
Valdes, A. M. et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann. Rheum. Dis. 70, 1556–1561 (2011).
Kerkhof, H. J. et al. Prediction model for knee osteoarthritis incidence, including clinical, genetic and biochemical risk factors. Ann. Rheum. Dis. 73, 2116–2121 (2014).
Zhang, W. et al. Nottingham knee osteoarthritis risk prediction models. Ann. Rheum. Dis. 70, 1599–1604 (2011).
Thomas, G. E. et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage 22, 1504–1510 (2014).
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196 (2012).
Oliveria, S. A., Felson, D. T., Reed, J. I., Cirillo, P. A. & Walker, A. M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 38, 1134–1141 (1995).
Englund, M. & Turkiewicz, A. Osteoarthritis increasingly common public disease [Swedish]. Lakartidningen 111, 930–931 (2014).
Hartvigsen, J., Davidsen, M., Søgaard, K., Roos, E. M. & Hestbaek, L. Self-reported musculoskeletal pain predicts long-term increase in general health care use: a population-based cohort study with 20-year follow-up. Scand. J. Public Health 42, 698–704 (2014).
Nüesch, E. et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 342, d1165 (2011).
Hawker, G. A. et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS ONE 9, e91286 (2014).
Barbour, K. E. et al. Hip osteoarthritis and the risk of all-cause and disease-specific mortality in older women: a population-based cohort study. Arthritis Rheumatol. 67, 1798–1805 (2015).
Arthritis Research Campaign. Osteoarthritis and obesity [online], (2009).
Ahluwalia, N. et al. Trends in overweight prevalence among 11-, 13- and 15-year-olds in 25 countries in Europe, Canada and USA from 2002 to 2010. Eur. J. Public Health 25 (Suppl. 2), 28–32 (2015).
Felson, D. T. et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 40, 728–733 (1997).
Lohmander, L. S., Gerhardsson de Verdier, M., Rollof, J., Nilsson, P. M. & Engström, G. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann. Rheum. Dis. 68, 490–496 (2009).
Gelber, A. C. et al. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am. J. Med. 107, 542–548 (1999).
Hart, D. J. & Spector, T. D. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J. Rheumatol. 20, 331–335 (1993).
Murphy, L. et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 59, 1207–1213 (2008).
Manninen, P., Riihimaki, H., Heliövaara, M. & Suomalainen, O. Weight changes and the risk of knee osteoarthritis requiring arthroplasty. Ann. Rheum. Dis. 63, 1434–1437 (2004).
Nicholls, A. S. et al. Change in body mass index during middle age affects risk of total knee arthoplasty due to osteoarthritis: a 19-year prospective study of 1003 women. Knee 19, 316–319 (2012).
Goulston, L. M. et al. Does obesity predict knee pain over fourteen years in women, independently of radiographic changes? Arthritis Care Res. (Hoboken) 63, 1398–1406 (2011).
McGoey, B. V., Deitel, M., Saplys, R. J. & Kliman, M. E. Effect of weight loss on musculoskeletal pain in the morbidly obese. J. Bone Joint Surg. Br. 72, 322–323 (1990).
Sommer, C. & Kress, M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 361, 184–187 (2004).
Sowers, M. F. et al. BMI vs. body composition and radiographically defined osteoarthritis of the knee in women: a 4-year follow-up study. Osteoarthritis Cartilage 16, 367–372 (2008).
Wluka, A. E., Lombard, C. B. & Cicuttini, F. M. Tackling obesity in knee osteoarthritis. Nat. Rev. Rheumatol. 9, 225–235 (2013).
Sacks, F. M. et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 360, 859–873 (2009).
Thorogood, A. et al. Isolated aerobic exercise and weight loss: a systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 124, 747–755 (2011).
Bray, G. A. Lifestyle and pharmacological approaches to weight loss: efficacy and safety. J. Clin. Endocrinol. Metab. 93, S81–S88 (2008).
Lombard, C., Deeks, A., Jolley, D., Ball, K. & Teede, H. A low intensity, community based lifestyle programme to prevent weight gain in women with young children: cluster randomised controlled trial. BMJ 341, c3215 (2010).
Appel, L. J. et al. Comparative effectiveness of weight-loss interventions in clinical practice. N. Engl. J. Med. 365, 1959–1968 (2011).
Lombard, C. B., Deeks, A. A. & Teede, H. J. A systematic review of interventions aimed at the prevention of weight gain in adults. Public Health Nutr. 12, 2236–2246 (2009).
Colquitt, J. L., Picot, J., Loveman, E. & Clegg, A. J. Surgery for obesity. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD003641. http://dx.doi.org/10.1002/14651858.CD003641.pub3.
Wing, R. R. & Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 82 (1 Suppl.), 222S–225S (2005).
Lim, J. Y., Tchai, E. & Jang, S. N. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: a randomized controlled trial. PMR 2, 723–731 (2010).
Christensen, R., Bartels, E. M., Astrup, A. & Bliddal, H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis. 66, 433–439 (2007).
Felson, D. T., Zhang, Y., Anthony, J. M., Naimark, A. & Anderson, J. J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann. Intern. Med. 116, 535–539 (1992).
Brown, T. D., Johnston, R. C., Saltzman, C. L., Marsh, J. L. & Buckwalter, J. A. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 20, 739–744 (2006).
Frobell, R. B. et al. The acutely ACL injured knee assessed by MRI: are large volume traumatic bone marrow lesions a sign of severe compression injury? Osteoarthritis Cartilage 16, 829–836 (2008).
Peat, G., Bergknut, C., Frobell, R., Jöud, A. & Englund, M. Population-wide incidence estimates for soft tissue knee injuries presenting to healthcare in southern Sweden: data from the Skåne Healthcare Register. Arthritis. Res. Ther. 16, R162 (2014).
Lohmander, L. S., Englund, P. M., Dahl, L. L. & Roos, E. M. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am. J. Sports Med. 35, 1756–1769 (2007).
Riordan, E. A., Little, C. & Hunter, D. Pathogenesis of post-traumatic OA with a view to intervention. Best Pract. Res. Clin. Rheumatol. 28, 17–30 (2014).
Richmond, S. A. et al. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J. Orthop. Sports Phys. Ther. 43, 515–524 (2013).
Øiestad, B. E., Engebretsen, L., Storheim, K. & Risberg, M. A. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am. J. Sports Med. 37, 1434–1443 (2009).
Muthuri, S. G., McWilliams, D. F., Doherty, M. & Zhang, W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage 19, 1286–1293 (2011).
Ingelsrud, L. H., Granan, L. P., Terwee, C. B., Engebretsen, L. & Roos, E. M. Proportion of patients reporting acceptable symptoms or treatment failure and their associated KOOS values at 6 to 24 months after anterior cruciate ligament reconstruction: a study from the Norwegian Knee Ligament Registry. Am. J. Sports Med. 43, 1902–1907 (2015).
Filbay, S. R., Ackerman, I. N., Russell, T. G., Macri, E. M. & Crossley, K. M. Health-related quality of life after anterior cruciate ligament reconstruction: a systematic review. Am. J. Sports Med. 42, 1247–1255 (2014).
Ardern, C. L., Webster, K. E., Taylor, N. F. & Feller, J. A. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br. J. Sports Med. 45, 596–606 (2011).
Grindem, H., Eitzen, I., Moksnes, H., Snyder-Mackler, L. & Risberg, M. A. A pair-matched comparison of return to pivoting sports at 1 year in anterior cruciate ligament-injured patients after a nonoperative versus an operative treatment course. Am. J. Sports Med. 40, 2509–2516 (2012).
Frobell, R. B. et al. Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ 346, f232 (2013).
Gagnier, J. J., Morgenstern, H. & Chess, L. Interventions designed to prevent anterior cruciate ligament injuries in adolescents and adults: a systematic review and meta-analysis. Am. J. Sports Med. 41, 1952–1962 (2013).
Myklebust, G., Skjølberg, A. & Bahr, R. ACL injury incidence in female handball 10 years after the Norwegian ACL prevention study: important lessons learned. Br. J. Sports Med. 47, 476–479 (2013).
Olympic.org, Official website of the Olympic Movement. Make sure your body is ready for exercise with 'Get Set'–an easy to use injury prevention app! [online], (2014).
FIFA. 11+, a complete warm-up programme [online], (2011).
Whittaker, J. L., Woodhouse, L. J., Nettel-Aguirre, A. & Emery, C. A. Outcomes associated with early post-traumatic osteoarthritis and other negative health consequences 3–10 years following knee joint injury in youth sport. Osteoarthritis Cartilage 23, 1122–1129 (2015).
Brophy, R. H., Gray, B. L., Nunley, R. M., Barrack, R. L. & Clohisy, J. C. Total knee arthroplasty after previous knee surgery: expected interval and the effect on patient age. J. Bone Joint Surg. Am. 96, 801–805 (2014).
Thorlund, J. B., Hare, K. B. & Lohmander, L. S. Large increase in arthroscopic meniscus surgery in the middle-aged and older population in Denmark from 2000 to 2011. Acta Orthop. 85, 287–292 (2014).
Englund, M., Roos, E. M. & Lohmander, L. S. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 48, 2178–2187 (2003).
Bedi, A. et al. Dynamic contact mechanics of the medial meniscus as a function of radial tear, repair, and partial meniscectomy. J. Bone Joint Surg. Am. 92, 1398–1408 (2010).
Ageberg, E. & Roos, E. M. Neuromuscular exercise as treatment of degenerative knee disease. Exerc. Sport Sci. Rev. 43, 14–22 (2015).
Ageberg, E., Nilsdotter, A., Kosek, E. & Roos, E. M. Effects of neuromuscular training (NEMEX-TJR) on patient-reported outcomes and physical function in severe primary hip or knee osteoarthritis: a controlled before-and-after study. BMC Musculoskelet. Disord. 14, 232 (2013).
Villadsen, A., Overgaard, S., Holsgaard-Larsen, A., Christensen, R. & Roos, E. M. Immediate efficacy of neuromuscular exercise in patients with severe osteoarthritis of the hip or knee: a secondary analysis from a randomized controlled trial. J. Rheumatol. 41, 1385–1394 (2014).
Bennell, K. L. et al. Neuromuscular versus quadriceps strengthening exercise in patients with medial knee osteoarthritis and varus malalignment: a randomized controlled trial. Arthritis Rheumatol. 66, 950–959 (2014).
Ageberg, E., Link, A. & Roos, E. M. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet. Disord. 11, 126 (2010).
Caspersen, C. J., Powell, K. E. & Christenson, G. M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 100, 126–131 (1985).
NCBI. Exercise Therapy [online], (2015).
Risberg, M. A., Holm, I., Myklebust, G. & Engebretsen, L. Neuromuscular training versus strength training during first 6 months after anterior cruciate ligament reconstruction: a randomized clinical trial. Phys. Ther. 87, 737–750 (2007).
Adams, D, Logerstedt, D. S., Hunter-Giordano, A., Axe, M. J. & Snyder-Mackler, L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J. Orthop. Sports Phys. Ther. 42, 601–614 (2012).
Øiestad, B. E., Juhl, C. B., Eitzen, I. & Thorlund, J. B. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 23, 171–177 (2015).
Frobell, R. B., Roos, E. M., Roos, H. P., Ranstam, J. & Lohmander, L. S. A randomized trial of treatment for acute anterior cruciate ligament tears. N. Engl. J. Med. 363, 331–342 (2010).
Roos, E. M. & Dahlberg, L. Positive effects of moderate exercise on knee cartilage glycosaminoglycan content. A four-month randomized controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 52, 3507–3514 (2005).
Moyer, R. F. et al. Combined effects of a valgus knee brace and lateral wedge foot orthotic on the external knee adduction moment in patients with varus gonarthrosis. Arch. Phys. Med. Rehabil. 94, 103–112 (2013).
Hunter, D. et al. Realignment treatment for medial tibiofemoral osteoarthritis: randomised trial. Ann. Rheum. Dis. 71, 1658–1665 (2012).
Fantini Pagani, C. H., Hinrichs, M. & Brüggemann, G. P. Kinetic and kinematic changes with the use of valgus knee brace and lateral wedge insoles in patients with medial knee osteoarthritis. J. Orthop. Res. 30, 1125–1132 (2012).
van Raaij, T. M., Reijman, M., Brouwer, R. W., Bierma-Zeinstra, S. M. & Verhaar, J. A. Medial knee osteoarthritis treated by insoles or braces: a randomized trial. Clin. Orthop. Relat. Res. 468, 1926–1932 (2010).
Brouwer, R. W., van Raaij, T. M., Verhaar, J. A., Coene, L. N. & Bierma-Zeinstra, S. M. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage 14, 777–783 (2006).
Moyer, R. F. et al. Biomechanical effects of valgus knee bracing: a systematic review and meta-analysis. Osteoarthritis Cartilage 23, 178–188 (2015).
Moyer, R. F. et al. Valgus bracing for knee osteoarthritis: a meta-analysis of randomized trials. Arthritis Care Res. (Hoboken) 67, 493–501 (2015).
Callaghan, M. J. et al. A randomised trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann. Rheum. Dis. 74, 1164–1170 (2015).
NHS England. Tools for shared decision making [online], (2015).
Petursdottir, U., Arnadottir, S. A. & Halldorsdottir, S. Facilitators and barriers to exercising among people with osteoarthritis: a phenomenological study. Phys. Ther. 90, 1014–1025 (2010).
Marks, R. Knee osteoarthritis and exercise adherence: a review. Curr. Aging Sci. 5, 72–83 (2012).
Bennell, K. L., Dobson, F. & Hinman, R. S. Exercise in osteoarthritis: moving from prescription to adherence. Best Pract. Res. Clin. Rheumatol. 28, 93–117 (2014).
Juhl, C., Christensen, R., Roos, E. M., Zhang, W. & Lund, H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 66, 622–636 (2014).
Bennell, K. L. et al. The influence of biomechanical characteristics on pain and function outcomes from exercise in medial knee osteoarthritis and varus malalignment: exploratory analyses from a randomised controlled trial. Arthritis Care Res. (Hoboken) http://dx.doi.org/10.1002/acr.22558.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article and contributed to discussion of content and writing the article, as well as reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
E.M.R. receives royalties for textbook chapters from Munksgaard and Studentlitteratur and is an associate editor of Osteoarthritis and Cartilage. N.K.A. has received honoraria from Bioberica, Flexion, GlaxoSmithKline, Novartis, Pfizer, Rottapharm, Servier and Smith and Nephew, held advisory board positions (which involved receipt of fees) with Bioventus, Merck Sharp and Dohme, Nicox and Q-MED, and received research grants from Bioberica, Roche and Servier.
Rights and permissions
About this article
Cite this article
Roos, E., Arden, N. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol 12, 92–101 (2016). https://doi.org/10.1038/nrrheum.2015.135
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.135
This article is cited by
-
Effects of a neuromuscular joint-protective exercise therapy program for treatment of wrist osteoarthritis: a randomized controlled trial
BMC Musculoskeletal Disorders (2024)
-
Detection of knee osteoarthritis based on recurrence quantification analysis, fuzzy entropy and shallow classifiers
Multimedia Tools and Applications (2024)
-
Association between physical activity and bone mineral density in postmenopausal women: a cross-sectional study from the NHANES 2007–2018
Journal of Orthopaedic Surgery and Research (2023)
-
Effects of backward walking exercise using lower body positive pressure treadmill on knee symptoms and physical function in individuals with knee osteoarthritis: a protocol for RCT
Journal of Orthopaedic Surgery and Research (2023)
-
The health and economic burden of musculoskeletal disorders in Belgium from 2013 to 2018
Population Health Metrics (2023)