Key Points
-
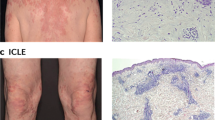
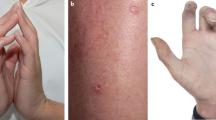
Skin injury is the second most common manifestation of systemic lupus erythematosus (SLE), yet its pathogenesis remains unclear
-
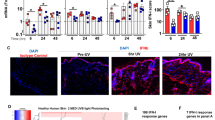
Ultraviolet light triggers activation of immune cells in areas where IgG has been deposited, which induces skin injury and disease flare
-
Mouse models of intradermal injection of lupus serum IgG develop skin inflammation and represent a useful tool to investigate the pathogenesis of skin injury in SLE
-
TNF receptor superfamily member 1A, tyrosine-protein kinase SYK, calcium/calmodulin-dependent protein kinase type IV and nuclear factor of activated T cells might be useful therapeutic targets to control skin injury in SLE
Abstract
Skin is the second most common organ (after the kidney) to be affected in patients with systemic lupus erythematosus (SLE), yet the aetiology of skin injury and the mechanisms involved in the development of dermal manifestations of SLE remain unclear. Ultraviolet light (UV), immune cells, cytokines and deposition of immunoglobulins all seem to have a role in the development of skin inflammation and damage in SLE. UV represents the most important environmental factor, and exposure to UV triggers the development of skin lesions in areas where immunoglobulin has been deposited and various other components of the immune system have accumulated. In addition, a number of intracellular kinases and transcription factors have also been demonstrated to be involved in the generation of skin lesions in lupus-prone mice. These molecules can be targeted by small-molecule inhibitors, leading to the prospect that treatments suitable for topical application, and with limited adverse effects, could be developed. Further studies to eliminate the burden of skin inflammation in patients with SLE are clearly required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Moulton, V. & Tsokos, G. C. T cell signaling abnormalities in systemic autoimmunity explain aberrant immune cell function and provide rational targets for treatment. J. Clin. Invest. (in press).
Cervera, R. et al. The European Working Party on Systemic Lupus Erythematosus. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore) 72, 113–124 (1993).
Lee, H. J. & Sinha, A. A. Cutaneous lupus erythematosus: understanding of clinical features, genetic basis, and pathobiology of disease guides therapeutic strategies. Autoimmunity 39, 433–444 (2006).
Kuhn, A. & Landmann, A. The classification and diagnosis of cutaneous lupus erythematosus. J. Autoimmun. 48–49, 14–19 (2014).
Privette, E. D. & Werth, V. P. Update on pathogenesis and treatment of CLE. Curr. Opin. Rheumatol. 25, 584–590 (2013).
Okon, L. G. & Werth, V. P. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract. Res. Clin. Rheumatol. 27, 391–404 (2013).
Biazar, C. et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun. Rev. 12, 444–454 (2013).
Gilliam, J. N. & Sontheimer, R. D. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J. Am. Acad. Dermatol. 4, 471–475 (1981).
Vera-Recabarren, M. A., García-Carrasco, M., Ramos-Casals, M. & Herrero, C. Comparative analysis of subacute cutaneous lupus erythematosus and chronic cutaneous lupus erythematosus: clinical and immunological study of 270 patients. Br. J. Dermatol. 162, 91–101 (2010).
Kuhn, A., Bein, D. & Bonsmann, G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun. Rev. 8, 441–448 (2009).
Provost, T. T. Lupus band test. Int. J. Dermatol. 20, 475–481 (1981).
Dahl, M. V. Usefulness of direct immunofluorescence in patients with lupus erythematosus. Arch. Dermatol. 119, 1010–1017 (1983).
Furukawa, F. et al. Dermatopathological studies on skin lesions of MRL mice. Arch. Dermatol. Res. 276, 186–194 (1984).
Deng, G. M. & Tsokos, G. C. Cholera toxin B accelerates disease progression in lupus-prone mice by promoting lipid raft aggregation. J. Immunol. 181, 4019–4026 (2008).
Kanauchi, H., Furukawa, F. & Imamura, S. Characterization of cutaneous infiltrates in MRL/lpr mice monitored from onset to the full development of lupus erythematosus-like skin lesions. J. Invest. Dermatol. 96, 478–483 (1991).
Menke, J. et al. Sunlight triggers cutaneous lupus through a CSF-1-dependent mechanism in MRL-Faslpr mice. J. Immunol. 181, 7367–7379 (2008).
Zahn, S. et al. Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Exp. Dermatol. 23, 516–518 (2014).
Kuhn, A., Wenzel, J. & Weyd, H. Photosensitivity, apoptosis, and cytokines in the pathogenesis of lupus erythematosus: a critical review. Clin. Rev. Allergy Immunol. 47, 148–162 (2014).
Yu C., Chang, C. & Zhang, J. Immunologic and genetic considerations of cutaneous lupus erythematosus: a comprehensive review. J. Autoimmun. 41, 34–45 (2013).
Yin, Q. et al. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity 47, 185–192 (2014).
Kirou, K. A. & Gkrouzman, E. Anti-interferon α treatment in SLE. Clin. Immunol. 148, 303–312 (2013).
Reefman, E., Kuiper, H., Limburg, P. C., Kallenberg, C. G. & Bijl, M. Type I interferons are involved in the development of ultraviolet B-induced inflammatory skin lesions in systemic lupus erythematosus patients. Ann. Rheum. Dis. 67, 11–18 (2008).
Kreuter, A. & Lehmann, P. Relevant new insights into the effects of photoprotection in cutaneous lupus erythematosus. Exp. Dermatol. 23, 712–713 (2014).
Sigges, J. et al. Therapeutic strategies evaluated by the European Society of Cutaneous Lupus Erythematosus (EUSCLE) Core Set Questionnaire in more than 1000 patients with cutaneous lupus erythematosus. Autoimmun. Rev. 12, 694–702 (2013).
Kuhn, A. et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br. J. Dermatol. 171, 571–579 (2014).
Rullo, O. J. & Tsao, B. P. Recent insights into the genetic basis of systemic lupus erythematosus. Ann. Rheum. Dis. 72 (Suppl. 2), ii56–ii61 (2013).
Sanchez, E. et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann. Rheum. Dis. 70, 1752–1757 (2011).
Tüngler, V., Silver, R. M., Walkenhorst, H., Günther, C. & Lee-Kirsch, M. A. Inherited or de novo mutation affecting aspartate 18 of TREX1 results in either familial chilblain lupus or Aicardi–Goutières syndrome. Br. J. Dermatol. 167, 212–214 (2012).
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Peng, S. L. et al. Murine lupus in the absence of αβ T cells. J. Immunol. 156, 4041–4049 (1996).
Peng, S. L., Madaio, M. P., Hayday, A. C. & Craft, J. Propagation and regulation of systemic autoimmunity by γδ T cells. J. Immunol. 157, 5689–5698 (1996).
Deng, G. M., Beltran, J., Chen, C., Terhorst, C. & Tsokos, G. C. T cell CD3ζ deficiency enables multiorgan tissue inflammation. J. Immunol. 191, 3563–3567 (2013).
Liossis, S. N., Ding, X. Z., Dennis, G. J. & Tsokos, G. C. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor ζ chain. J. Clin. Invest. 101, 1448–1457 (1998).
Li, Y. et al. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J. Immunol. 178, 1938–1947 (2007).
Norman, M. U., James, W. G. & Hickey, M. J. Differential roles of ICAM-1 and VCAM-1 in leukocyte-endothelial cell interactions in skin and brain of MRL/faslpr mice. J. Leukoc. Biol. 84, 68–76 (2008).
Peng, S. L. et al. αβ T cell regulation and CD40 ligand dependence in murine systemic autoimmunity. J. Immunol. 158, 2464–2470 (1997).
Kinoshita, K. et al. Costimulation by B7–1 and B7–2 is required for autoimmune disease in MRL-Faslpr mice. J. Immunol. 164, 6046–6056 (2000).
Chan, O. T., Madaio, M. P. & Shlomchik, M. J. The central and multiple roles of B cells in lupus pathogenesis. Immunol. Rev. 169, 107–121 (1999).
Lenda, D. M., Stanley, E. R. & Kelley, V. R. Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Faslpr mice. J. Immunol. 173, 4744–4754 (2004).
Teichmann, L. L. et al. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity 33, 967–978 (2010).
Blomberg, S. et al. Presence of cutaneous interferon-α producing cells in patients with systemic lupus erythematosus. Lupus 10, 484–490 (2001).
Farkas, L., Beiske, K., Lund-Johansen, F., Brandtzaeg, P. & Jahnsen, F. L. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 (2001).
Guiducci, C. et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 207, 2931–2942 (2010).
Sisirak, V. et al. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 211, 1969–1976 (2014).
Rowland, S. L. et al. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 211, 1977–1991 (2014).
Yang, J. Q. et al. CD1d deficiency exacerbates inflammatory dermatitis in MRL-lpr/lpr mice. Eur. J. Immunol. 34, 1723–1732 (2004).
Eriksson, A. U. & Singh, R. R. Cutting edge: migration of Langerhans dendritic cells is impaired in autoimmune dermatitis. J. Immunol. 181, 7468–7472 (2008).
Hochrein, H., O'Keeffe, M. & Wagner, H. Human and mouse plasmacytoid dendritic cells. Hum. Immunol. 63, 1103–1110 (2002).
Deng, G. M., Liu, L., Kyttaris, V. C. & Tsokos, G. C. Lupus serum IgG induces skin inflammation through the TNFR1 signaling pathway. J. Immunol. 184, 7154–7161 (2010).
Deng, G. M., Nilsson, M., Verdrengh, M., Collins, L. V. & Tarkowski, A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 5, 702–705 (1999).
Deng, G. M., Liu, Z. Q. & Tarkowski, A. Intracisternally localized bacterial DNA containing CpG motifs induces meningitis. J. Immunol. 167, 4616–4626 (2001).
Deng, G. M., Verdrengh, M., Liu, Z. Q. & Tarkowski, A. The major role of macrophages and their product tumor necrosis factor α in the induction of arthritis triggered by bacterial DNA containing CpG motifs. Arthritis Rheum. 43, 2283–2289 (2000).
Berden, J. H., Licht, R., van Bruggen, M. C. & Tax, W. J. Role of nucleosomes for induction and glomerular binding of autoantibodies in lupus nephritis. Curr. Opin. Nephrol. Hypertens. 8, 299–306 (1999).
Kalaaji, M. et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 71, 664–672 (2007).
Kalaaji, M., Mortensen, E., Jørgensen, L., Olsen, R. & Rekvig, O. P. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am. J. Pathol. 168, 1779–1792 (2006).
Mostoslavsky, G. et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur. J. Immunol. 31, 1221–1227 (2001).
Deocharan, B., Qing, X., Lichauco, J. & Putterman, C. α-Actinin is a cross-reactive renal target for pathogenic anti-DNA antibodies. J. Immunol. 168, 3072–3078 (2002).
DeGiorgio, L. A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001).
Lee, L. A., Gaither, K. K., Coulter, S. N., Norris, D. A. & Harley, J. B. Pattern of cutaneous immunoglobulin G deposition in subacute cutaneous lupus erythematosus is reproduced by infusing purified anti-Ro (SSA) autoantibodies into human skin-grafted mice. J. Clin. Invest. 83, 1556–1562 (1989).
Shi, Z. R. et al. Association of anti-acidic ribosomal protein p0 and anti-galectin 3 antibodies with the development of skin lesions in systemic lupus erythematosus. Arthritis Rheumatol. 67, 193–203 (2015).
Oke, V. et al. High Ro52 expression in spontaneous and UV-induced cutaneous inflammation. J. Invest. Dermatol. 129, 2000–2010 (2009).
Espinosa, A. et al. Loss of the lupus autoantigen Ro52/TRIM21 induces tissue inflammation and systemic autoimmunity by dysregulating the IL-23–TH17 pathway. J. Exp. Med. 206, 1661–1671 (2009).
Haas, C., Ryffel, B. & Le Hir, M. IFN-γ receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW) F1 mice. J. Immunol. 160, 3713–3718 (1998).
Schwarting, A., Wada, T., Kinoshita, K., Tesch, G. & Kelley, V. R. IFN-γ receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Faslpr mice. J. Immunol. 161, 494–503 (1998).
Kikawada, E., Lenda, D. M. & Kelley, V. R. IL-12 deficiency in MRL-Faslpr mice delays nephritis and intrarenal IFN-γ expression, and diminishes systemic pathology. J. Immunol. 170, 3915–3925 (2003).
Wenzel, J., Zahn, S., Bieber, T. & Tüting, T. Type I interferon-associated cytotoxic inflammation in cutaneous lupus erythematosus. Arch. Dermatol. Res. 301, 83–86 (2009).
Zampieri, S. et al. Tumour necrosis factor α is expressed in refractory skin lesions from patients with subacute cutaneous lupus erythematosus. Ann. Rheum. Dis. 65, 545–548 (2006).
Aringer, M. & Smolen, J. S. Therapeutic blockade of TNF in patients with SLE—promising or crazy? Autoimmun. Rev. 11, 321–325 (2012).
Postal, M. & Appenzeller, S. The role of tumor necrosis factor-α (TNF-α) in the pathogenesis of systemic lupus erythematosus. Cytokine 56, 537–543 (2011).
Deng, G. M., Zheng, L., Chan, F. K. & Lenardo, M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat. Med. 11, 1066–1072 (2005).
Chan, F. K. et al. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288, 2351–2354 (2000).
Deng, G. M., Liu, L. & Tsokos, G. C. Targeted tumor necrosis factor receptor I preligand assembly domain improves skin lesions in MRL/lpr mice. Arthritis Rheum. 62, 2424–2431 (2010).
Zhou, T. et al. Greatly accelerated lymphadenopathy and autoimmune disease in lpr mice lacking tumor necrosis factor receptor I. J. Immunol. 156, 2661–2665 (1996).
Kurosaki, T. et al. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J. Exp. Med. 179, 1725–1729 (1994).
Pamuk, O. N. & Tsokos, G. C. Spleen tyrosine kinase inhibition in the treatment of autoimmune, allergic and autoinflammatory diseases. Arthritis Res. Ther. 12, 222 (2010).
Deng, G. M., Liu, L., Bahjat, F. R., Pine, P. R. & Tsokos, G. C. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum. 62, 2086–2092 (2010).
Nazareth, M. et al. Altered Bax expression and decreased apoptosis in bone marrow cells of lupus-susceptible NZB/W mice. Lupus 10, 785–793 (2001).
Takeuchi, O. et al. Essential role of BAX, BAK in B cell homeostasis and prevention of autoimmune disease. Proc. Natl Acad. Sci. USA 102, 11272–11277 (2005).
Lu, T. Y. et al. A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at University College London Hospital: the first fifty patients. Arthritis Rheum. 61, 482–487 (2009).
Terrier, B. et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 62, 2458–2466 (2010).
Hofmann, S. C., Leandro, M. J., Morris, S. D. & Isenberg, D. A. Effects of rituximab-based B-cell depletion therapy on skin manifestations of lupus erythematosus—report of 17 cases and review of the literature. Lupus 22, 932–939 (2013).
Hook, S. S. & Means, A. R. Ca2+/CaM-dependent kinases: from activation to function. Annu. Rev. Pharmacol. Toxicol. 41, 471–505 (2001).
Soderling, T. R. The Ca–calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 24, 232–236 (1999).
Juang, Y. T. et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J. Clin. Invest. 115, 996–1005 (2005).
Ichinose, K., Juang, Y. T., Crispín, J. C., Kis-Toth, K. & Tsokos, G. C. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis Rheum. 63, 523–529 (2011).
Müller, M. R. & Rao, A. FAT, immunity and cancer: a transcription factor comes of age. Nat. Rev. Immunol. 10, 645–656 (2010).
Shaw, J. P. et al. Identification of a putative regulator of early T cell activation genes. Science 241, 202–205 (1988).
Hogan, P. G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 (2003).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Zanoni, I. et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460, 264–268 (2009).
Shukla, U., Hatani, T., Nakashima, K., Ogi, K. & Sada, K. Tyrosine phosphorylation of 3BP2 regulates B cell receptor-mediated activation of NFAT. J. Biol. Chem. 284, 33719–33728 (2009).
Crist, S. A., Sprague, D. L. & Ratliff, T. L. Nuclear factor of activated T cells (NFAT) mediates CD154 expression in megakaryocytes. Blood 111, 3553–3561 (2008).
Kyttaris, V. C., Wang, Y., Juang, Y. T., Weinstein, A. & Tsokos, G. C. Increased levels of NF-ATc2 differentially regulate CD154 and IL-2 genes in T cells from patients with systemic lupus erythematosus. J. Immunol. 178, 1960–1966 (2007).
Kyttaris, V. C., Zhang, Z., Kampagianni, O. & Tsokos, G. C. Calcium signaling in systemic lupus erythematosus T cells: a treatment target. Arthritis Rheum. 63, 2058–2066 (2011).
Jain, J. et al. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 365, 352–355 (1993).
McCaffrey, P. G. et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science 262, 750–754 (1993).
Mulero, M. C. et al. Inhibiting the calcineurin-NFAT (nuclear factor of activated T cells) signaling pathway with a regulator of calcineurin-derived peptide without affecting general calcineurin phosphatase activity. J. Biol. Chem. 284, 9394–9401 (2009).
Tzung, T. Y., Liu, Y. S., & Chang, H. W. Tacrolimus vs. clobetasol propionate in the treatment of facial cutaneous lupus erythematosus: a randomized, double-blind, bilateral comparison study. Br. J. Dermatol. 156, 191–192 (2007).
Kuhn A. et al. Efficacy of tacrolimus 0.1% ointment in cutaneous lupus erythematosus: a multicenter, randomized, double-blind, vehicle-controlled trial. J. Am. Acad. Dermatol. 65, 54–64 (2011).
Lo, M. S. & Tsokos, G. C. Treatment of systemic lupus erythematosus: new advances in targeted therapy. Ann. NY Acad. Sci. 1247, 138–152 (2012).
Acknowledgements
The authors acknowledge Health & Human Sciences–NIH–National Institute of Allergy and Infectious Diseases (NIAID) grant R01 AI 42269 to G.C.T.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the manuscript (researching data for the article, discussions of its content, writing, review and editing of the manuscript before submission).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Deng, GM., Tsokos, G. Pathogenesis and targeted treatment of skin injury in SLE. Nat Rev Rheumatol 11, 663–669 (2015). https://doi.org/10.1038/nrrheum.2015.106
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.106
This article is cited by
-
Skin–kidney crosstalk in SLE
Nature Reviews Rheumatology (2021)
-
Altered expression of genes controlling metabolism characterizes the tissue response to immune injury in lupus
Scientific Reports (2021)
-
Autoimmunity and organ damage in systemic lupus erythematosus
Nature Immunology (2020)
-
Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity
Nature Communications (2020)
-
Highly selective inhibition of Bruton’s tyrosine kinase attenuates skin and brain disease in murine lupus
Arthritis Research & Therapy (2018)