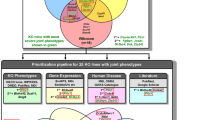
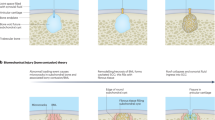
Key Points
-
Osteoarthritis (OA) imposes huge social and economic burdens on our society, causing pain and disability as well as reduced quality of life in individuals with the condition
-
Owing to sophisticated genetic engineering strategies, mouse models are of great value in understanding the role of individual genes in joint health and in the development of OA
-
Individual risk factors for OA (injury, diet, activity, genetics, and so on) can be individually modified in mouse models of the disease
-
New assessment tools (in particular for pain and activity) enable the examination of patient-relevant parameters in mouse models of OA
-
High-throughput 'omics' approaches coupled to the use of genetically altered mice will assist the comprehensive understanding of physiology and pathophysiology of joint tissues
Abstract
Osteoarthritis (OA) is a prevalent musculoskeletal disease that results in pain and low quality of life for patients, as well as enormous medical and socioeconomic burdens. The molecular mechanisms responsible for the initiation and progression of OA are still poorly understood. As such, mouse models of the disease are having increasingly important roles in OA research owing to the advancements of microsurgical techniques and the use of genetically modified mice, as well as the development of novel assessment tools. In this Review, we discuss available mouse models of OA and applicable assessment tools in studies of experimental OA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Murphy, L. & Helmick, C. G. The impact of osteoarthritis in the United States: a population-health perspective. Am. J. Nurs. 112 (Suppl. 1), S13–S19 (2012).
Grynpas, D. M. D., Alpert, B., Katz, I., Lieberman, I. & Pritzker, K. P. H. Subchondral bone in osteoarthritis. Calcif. Tissue Int. 49, 20–26 (1991).
Brandt, K. D. Osteoarthritis clinical trials have not identified efficacious therapies because traditional imaging outcome measures are inadequate. Arthritis Rheum. http://dx.doi.org/10.1002/art.38084.
Little, C. B. & Hunter, D. J. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat. Rev. Rheumatol. 9, 485–497 (2013).
Tochigi, Y. et al. Instability dependency of osteoarthritis development in a rabbit model of graded anterior cruciate ligament transection. J. Bone Joint Surg. Am. 93, 640–647 (2011).
Kamekura, S. et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage 13, 632–641 (2005).
Knights, C. B., Gentry, C. & Bevan, S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain 153, 281–292 (2012).
Glasson, S. S. et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 50, 2547–2558 (2004).
Glasson, S. S. et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434, 644–648 (2005).
Welch, I. D., Cowan, M. F., Beier, F. & Underhill, T. M. The retinoic acid binding protein CRABP2 is increased in murine models of degenerative joint disease. Arthritis Res. Ther. 11, R14 (2009).
Miller, R. E. et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc. Natl Acad. Sci. USA 109, 20602–20607 (2012).
Loeser, R. F. et al. Disease progression and phasic changes in gene expression in a mouse model of osteoarthritis. PLoS ONE 8, e54633 (2013).
Loeser, R. F. et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 64, 705–717 (2012).
Appleton, C. T. G., Pitelka, V., Henry, J. & Beier, F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 56, 1854–1868 (2007).
Ma, H.-L. et al. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage 15, 695–700 (2007).
Centers for Disease Control and Prevention (CDC). Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2007–2009. MMWR Morb. Mortal. Wkly Rep. 59, 1261–1265 (2010).
Ameye, L. G. & Young, M. F. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr. Opin. Rheumatol. 18, 537–547 (2006).
Little, C. B. & Zaki, S. What constitutes an 'animal model of osteoarthritis'—the need for consensus? Osteoarthritis Cartilage 20, 261–267 (2012).
Lotz, M. K. & Kraus, V. B. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res. Ther. 12, 211 (2010).
Christiansen, B. A. et al. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 20, 773–782 (2012).
Lockwood, K. A., Chu, B. T., Anderson, M. J., Haudenschild, D. R. & Christiansen, B. A. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J. Orthop. Res. 32, 79–88 (2014).
Furman, B. D. et al. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J. Orthop. Res. 25, 578–592 (2007).
Lewis, J. S. et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage 19, 864–873 (2011).
Ward, B. D. et al. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 58, 744–753 (2008).
Louer, C. R. et al. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum. 64, 3220–3230 (2012).
Galois, L. et al. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: a pilot study. Osteoarthritis Cartilage 12, 779–786 (2004).
Huang, M. H., Lin, Y. S., Yang, R. C. & Lee, C. L. A comparison of various therapeutic exercises on the functional status of patients with knee osteoarthritis. Semin. Arthritis Rheum. 32, 398–406 (2003).
Manninen, P., Riihimaki, H., Heliovaara, M. & Suomalainen, O. Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxford) 40, 432–437 (2001).
De Souza, R. L. et al. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37, 810–818 (2005).
Poulet, B., Hamilton, R. W., Shefelbine, S. & Pitsillides, A. A. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 63, 137–147 (2010).
Poulet, B., Westerhof, T. A. T., Hamilton, R. W., Shefelbine, S. J. & Pitsillides, A. A. Spontaneous osteoarthritis in Str/ort mice is unlikely due to greater vulnerability to mechanical trauma. Osteoarthritis Cartilage 21, 756–763 (2013).
Ko, F. C. et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 65, 1569–1578 (2013).
Wilhelm, G. & Faust, R. Suitability of the C57 black mouse as an experimental animal for the study of skeletal changes due to ageing, with special reference to osteo-arthrosis and its response to tribenoside. Pharmacology 14, 289–296 (1976).
Walton, M. Degenerative joint disease in the mouse knee; histological observations. J. Pathol. 123, 109–122 (1977).
Poulet, B. et al. Time-series transcriptional profiling yields new perspectives on susceptibility to murine osteoarthritis. Arthritis Rheum. 64, 3256–3266 (2012).
Sokoloff, L., Crittenden, L. B., Yamamoto, R. S. & Jay, G. E. The genetics of degenerative joint disease in mice. Arthritis Rheum. 5, 531–546 (1962).
Säämänen, A. K. et al. Osteoarthritis-like lesions in transgenic mice harboring a small deletion mutation in type II collagen gene. Osteoarthritis Cartilage 8, 248–257 (2000).
Hu, K. et al. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 54, 2891–2900 (2006).
Neuhold, L. A. et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Invest. 107, 35–44 (2001).
Pitsillides, A. A. & Beier, F. Cartilage biology in osteoarthritis—lessons from developmental biology. Nat. Rev. Rheumatol. 7, 654–663 (2011).
Blagojevic, M., Jinks, C., Jeffery, A. & Jordan, K. P. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 18, 24–33 (2010).
Griffin, T. M., Huebner, J. L., Kraus, V. B., Yan, Z. & Guilak, F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheum. 64, 443–453 (2012).
Phan, M. N. et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 60, 3028–3037 (2009).
O'Conor, C. J., Leddy, H. A., Benefield, H. C., Liedtke, W. B. & Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl Acad. Sci. USA 111, 1316–1321 (2014).
O'Conor, C. J., Griffin, T. M., Liedtke, W. & Guilak, F. Increased susceptibility of Trpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann. Rheum. Dis. 72, 300–304 (2013).
Griffin, T. M., Huebner, J. L., Kraus, V. B. & Guilak, F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 60, 2935–2944 (2009).
Mooney, R. A., Sampson, E. R., Lerea, J., Rosier, R. N. & Zuscik, M. J. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res. Ther. 13, R198 (2011).
Guzman, R. E., Evans, M. G., Bove, S., Morenko, B. & Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol. Pathol. 31, 619–624 (2003).
Barve, R. A. et al. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthritis Cartilage 15, 1190–1198 (2007).
Gong, D. et al. Mechanisms of olive leaf extract-ameliorated rat arthritis caused by kaolin and carrageenan. Phytother. Res. 26, 397–402 (2012).
Budsberg, S. C. et al. Effect of perzinfotel and a proprietary phospholipase A(2) inhibitor on kinetic gait and subjective lameness scores in dogs with sodium urate-induced synovitis. Am. J. Vet. Res. 72, 757–763 (2011).
VANOSCH, G. et al. Induction of osteoarthritis by intra-articular injection of collagenase in mice. Strain and sex related differences. Osteoarthritis Cartilage 1, 171–177 (1993).
Jevsevar, D. S. et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on treatment of osteoarthritis of the knee, 2nd Edition. J. Bone Joint Surg. Am. 95, 1885–1886 (2013).
Watters, J. W. et al. Inverse relationship between matrix remodeling and lipid metabolism during osteoarthritis progression in the STR/Ort mouse. Arthritis Rheum. 56, 2999–3009 (2007).
McNulty, M. A. et al. Histopathology of naturally occurring and surgically induced osteoarthritis in mice. Osteoarthritis Cartilage 20, 949–956 (2012).
Glasson, S. S., Chambers, M. G., van den Berg, W. B. & Little, C. B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18 (Suppl. 3), S17–S23 (2010).
Moussavi-Harami, S. F., Pedersen, D. R., Martin, J. A., Hillis, S. L. & Brown, T. D. Automated objective scoring of histologically apparent cartilage degeneration using a custom image analysis program. J. Orthop. Res. 27, 522–528 (2009).
Orth, P., Zurakowski, D., Wincheringer, D. & Madry, H. Reliability, reproducibility, and validation of five major histological scoring systems for experimental articular cartilage repair in the rabbit model. Tissue Eng. Part C Methods 18, 329–339 (2012).
Zhang, R. et al. Gene expression analyses of subchondral bone in early experimental osteoarthritis by microarray. PLoS ONE 7, e32356 (2012).
Burleigh, A. et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 64, 2278–2288 (2012).
Bonner, R. F. et al. Laser capture microdissection: molecular analysis of tissue. Science 278, 1481–1483 (1997).
Espina, V. et al. Laser-capture microdissection. Nat. Protoc. 1, 586–603 (2006).
Ikeda, K. et al. Extraction and analysis of diagnostically useful proteins from formalin-fixed, paraffin-embedded tissue sections. J. Histochem. Cytochem. 46, 397–403 (1998).
Scicchitano, M. S., Dalmas, D. A., Boyce, R. W., Thomas, H. C. & Frazier, K. S. Protein extraction of formalin-fixed, paraffin-embedded tissue enables robust proteomic profiles by mass spectrometry. J. Histochem. Cytochem. 57, 849–860 (2009).
Mobasheri, A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthritis Cartilage 20, 1451–1464 (2012).
Braza-Boïls, A., Ferrándiz, M. L., Terencio, M. C. & Alcaraz, M. J. Analysis of early biochemical markers and regulation by tin protoporphyrin IX in a model of spontaneous osteoarthritis. Exp. Gerontol. 47, 406–409 (2012).
Radin, E. L. & Rose, R. M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Rel. Res. 213, 34–40 (1986).
Zhen, G. et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19, 704–712 (2013).
Botter, S. M. et al. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology 43, 379–388 (2006).
D'Souza, W. N., Ng, G. Y., Youngblood, B. D., Tsuji, W. & Lehto, S. G. A review of current animal models of osteoarthritis pain. Curr. Pharm. Biotechnol. 12, 1596–1612 (2011).
Malfait, A. M., Little, C. B. & McDougall, J. J. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis Cartilage 21, 1316–1326 (2013).
Inglis, J. J. et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 58, 3110–3119 (2008).
Van de Weerd, H. A. et al. Validation of a new system for the automatic registration of behaviour in mice and rats. Behav. Processes 53, 11–20 (2001).
Malfait, A. M. et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage 18, 572–580 (2010).
Botter, S. M. et al. ADAMTS5−/− mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage 17, 636–645 (2009).
Vogelaar, C. F. et al. Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res. 1027, 67–72 (2004).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Bush, J. R. & Beier, F. TGF-β and osteoarthritis—the good and the bad. Nat. Med. 19, 667–669 (2013).
Plaas, A. et al. Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis. Arthritis Res. Ther. 13, R46 (2011).
Costello, K. E., Guilak, F., Setton, L. A. & Griffin, T. M. Locomotor activity and gait in aged mice deficient for type IX collagen. J. Appl. Physiol. 109, 211–218 (2010).
Eggli, P. S., Hunzinker, E. B. & Schenk, R. K. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medical femoral condyles in young adult rabbits. Anat. Rec. 222, 217–227 (1988).
Kim, B. J. et al. Establishment of a reliable and reproducible murine osteoarthritis model. Osteoarthritis Cartilage 21, 2013–2020 (2013).
Ruan, M. Z. C. et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 5, 176ra34 (2013).
Lai, Y. et al. ADAMTS-7 forms a positive feedback loop with TNF-α in the pathogenesis of osteoarthritis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2013-203561.
Acknowledgements
The authors would like to acknowledge funding support received from the Canadian Institutes of Health Research and Canada Research Chairs, as well as the China Scholarship Council for supporting the visit by F.H. to Canada. We also thank all members of the Beier Laboratory for fruitful discussions.
Author information
Authors and Affiliations
Contributions
Both authors made equal contributions to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fang, H., Beier, F. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat Rev Rheumatol 10, 413–421 (2014). https://doi.org/10.1038/nrrheum.2014.46
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.46
This article is cited by
-
A mutation in the ZNF687 gene that is responsible for the severe form of Paget’s disease of bone causes severely altered bone remodeling and promotes hepatocellular carcinoma onset in a knock-in mouse model
Bone Research (2023)
-
An injectable copolymer of fatty acids (ARA 3000 BETA) as a promising treatment for osteoarthritis
Scientific Reports (2023)
-
Effect of intra-articular injection of a hyaluronic acid-alendronate conjugate on post-traumatic osteoarthritis induced by destabilization of the medial meniscus in rats
Scientific Reports (2023)
-
Ligamentous injury-induced ankle instability causing posttraumatic osteoarthritis in a mouse model
BMC Musculoskeletal Disorders (2022)
-
New imaging tools for mouse models of osteoarthritis
GeroScience (2022)