Key Points
-
The search for biomarkers in paediatric rheumatic diseases is attracting increased interest
-
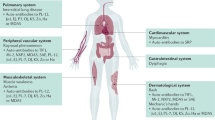
Several biomarkers have potential to predict the clinical phenotype, disease activity, response to treatment and course of disease in juvenile idiopathic arthritis
-
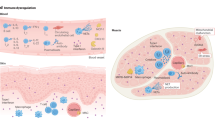
Biomarkers that reflect the degree of activation and expansion of T cells and macrophages could help to identify subclinical macrophage activation syndrome
-
Novel urine biomarkers for childhood lupus nephritis hold promise for facilitating early diagnosis, improving disease monitoring and assessment of response to therapy
-
Myositis-specific autoantibodies define distinct serological subgroups of juvenile idiopathic inflammatory myositis, albeit with similar clinical characteristics
-
The diagnostic power of biomarkers might help to avoid invasive procedures, such as renal biopsy in systemic lupus erythematosus, and muscle biopsy in juvenile dermatomyositis
Abstract
The search for biomarkers in paediatric rheumatic diseases, particularly juvenile idiopathic arthritis (JIA), childhood lupus nephritis (LN), and juvenile idiopathic inflammatory myopathies (JIIMs) is attracting increased interest. In JIA, a number of biomarkers have shown potential for predicting clinical phenotype, disease activity and severity, clinical remission and relapse, response to treatment, and disease course over time. In systemic JIA, measurement of biomarkers that reflect the degree of activation and expansion of T cells and macrophages might be helpful for detecting subclinical macrophage activation syndrome. Urine biomarkers for childhood LN hold promise for facilitating early diagnosis and improving disease monitoring and assessment of response to therapy. Myositis-specific autoantibodies define distinct serological subgroups of JIIMs, albeit with similar clinical features, responses to therapy, and prognoses. Use of biomarkers may potentially help to avoid invasive procedures, such as renal biopsy in systemic lupus erythematosus and muscle biopsy in juvenile dermatomyositis. Incorporation of effective and reliable biomarkers into routine practice might facilitate adoption of a stratified approach to investigation and management, foster the implementation of research into the design of personalized and targeted therapies, and ultimately lead to more rational and effective clinical care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
De Gruttola, V. G. et al. Considerations in the evaluation of surrogate endpoints in clinical trials. Summary of a National Institutes of Health workshop. Control Clin. Trials 22, 485–502 (2001).
Ahearn, J. M., Liu, C. C., Kao, A. H. & Manzi, S. Biomarkers for systemic lupus erythematosus. Transl. Res. 159, 326–342 (2012).
Bennett, M. & Brunner, H. I. Biomarkers and updates on pediatric lupus nephritis. Rheum. Dis. Clin. North Am. 39, 833–853 (2013).
Klassen, T. P., Hartling, L., Craig, J. C. & Offringa, M. Children are not just small adults: the urgent need for high-quality trial evidence in children. PLoS Med. 5, e172 (2008).
Goldman, J., Becker, M. L., Jones, B., Clements, M. & Leeder, J. S. Development of biomarkers to optimize pediatric patient management: what makes children different? Biomark. Med. 5, 781–794 (2011).
Duurland, C. L. & Wedderburn, L. R. Current developments in the use of biomarkers for juvenile idiopathic arthritis. Curr. Rheumatol. Rep. 16, 406 (2014).
Petty, R. E. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 31, 390–392 (2004).
Ravelli, A. & Martini, A. Juvenile idiopathic arthritis. Lancet 369, 767–778 (2007).
Prakken, B., Albani, S. & Martini, A. Juvenile idiopathic arthritis. Lancet 377, 2138–2149 (2011).
Southwood, T. R. & Ryder, C. A. Ophthalmological screening in juvenile arthritis: should the frequency of screening be based on the risk of developing chronic iridocyclitis? Br. J. Rheumatol. 31, 633–634 (1992).
American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology: Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics 92, 295–296 (1993).
Cassidy, J., Kivlin, J., Lindsley, C. & Nocton, J. Ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics 117, 1843–1845 (2006).
Heiligenhaus, A., Niewerth, M., Ganser, G., Heinz, C. & Minden, K. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford) 46, 1015–1019 (2007).
Calandra, S. et al. Female sex and oligoarthritis category are not risk factors for uveitis in Italian children with juvenile idiopathic arthritis. J. Rheumatol. 41, 1416–1425 (2014).
Hunter, P. J. et al. Biologic predictors of extension of oligoarticular juvenile idiopathic arthritis as determined from synovial fluid cellular composition and gene expression. Arthritis Rheum. 62, 896–907 (2010).
Gibson, D. S. et al. Stratification and monitoring of juvenile idiopathic arthritis patients by synovial proteome analysis. J. Proteome Res. 8, 5601–5609 (2009).
Myles, A. & Aggarwal, A. Expression of Toll-like receptors 2 and 4 is increased in peripheral blood and synovial fluid monocytes of patients with enthesitis-related arthritis subtype of juvenile idiopathic arthritis. Rheumatology (Oxford) 50, 481–488 (2011).
Viswanath, V., Myles, A., Dayal, R. & Aggarwal, A. Levels of serum matrix metalloproteinase-3 correlate with disease activity in the enthesitis-related arthritis category of juvenile idiopathic arthritis. J. Rheumatol. 38, 2482–2487 (2011).
Aoki, C. et al. Discrepancy between clinical and radiological responses to tocilizumab treatment in patients with systemic-onset juvenile idiopathic arthritis. J. Rheumatol. 41, 1171–1177 (2014).
Foell, D. & Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 50, 3762–3771 (2004).
Holzinger, D. et al. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 71, 974–980 (2012).
Vastert, S. & Prakken, B. Update on research and clinical translation on specific clinical areas: from bench to bedside: how insight in immune pathogenesis can lead to precision medicine of severe juvenile idiopathic arthritis. Best. Pract. Res. Clin. Rheumatol. 28, 229–246 (2014).
de Jager, W. et al. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann. Rheum. Dis. 66, 589–598 (2007).
Lotito, A. P., Campa, A., Silva, C. A., Kiss, M. H. & Mello, S. B. Interleukin 18 as a marker of disease activity and severity in patients with juvenile idiopathic arthritis. J. Rheumatol. 34, 823–830 (2007).
Ling, X. B. et al. Urine peptidomic and targeted plasma protein analyses in the diagnosis and monitoring of systemic juvenile idiopathic arthritis. Clin. Proteomics 6, 175–193 (2010).
Scott, C. et al. A reappraisal of intra-articular corticosteroid therapy in juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 28, 774–781 (2010).
Lanni, S. et al. Outcome and predicting factors of single and multiple intra-articular corticosteroid injections in children with juvenile idiopathic arthritis. Rheumatology (Oxford) 50, 1627–1634 (2011).
Vilca, I. et al. Predictors of poor response to methotrexate in polyarticular-course juvenile idiopathic arthritis: analysis of the PRINTO methotrexate trial. Ann. Rheum. Dis. 69, 1479–1483 (2010).
Papsdorf, V. & Horneff, G. Complete control of disease activity and remission induced by treatment with etanercept in juvenile idiopathic arthritis. Rheumatology (Oxford) 50, 214–221 (2011).
Otten, M. H. et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA 306, 2340–2347 (2011).
Solari, N. et al. Factors associated with achievement of inactive disease in children with juvenile idiopathic arthritis treated with etanercept. J. Rheumatol. 40, 192–200 (2013).
Gattorno, M. et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 58, 1505–1515 (2008).
Moncrieffe, H. et al. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 52, 1467–1476 (2013).
Vastert, S. J. et al. Effectiveness of first-line treatment with recombinant interleukin-1 receptor antagonist in steroid-naive patients with new-onset systemic juvenile idiopathic arthritis: results of a prospective cohort study. Arthritis Rheumatol. 66, 1034–1043 (2014).
Foell, D. et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA 303, 1266–1273 (2010).
Gerss, J. et al. Phagocyte-specific S100 proteins and high-sensitivity C reactive protein as biomarkers for a risk-adapted treatment to maintain remission in juvenile idiopathic arthritis: a comparative study. Ann. Rheum. Dis. 71, 1991–1997 (2012).
Rothmund, F. et al. Validation of relapse risk biomarkers for routine use in patients with juvenile idiopathic arthritis. Arthritis Care Res. (Hoboken) 66, 949–955 (2013).
Ravelli, A., Grom, A. A., Behrens, E. M. & Cron, R. Q. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 13, 289–298 (2012).
Behrens, E. M., Beukelman, T., Paessler, M. & Cron, R. Q. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J. Rheumatol. 34, 1133–1138 (2007).
Bleesing, J. et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 56, 965–971 (2007).
Ho, C. et al. Marrow assessment for haemophagocytic lymphohistiocytosis demonstrates poor correlation with disease probability. Am. J. Clin. Pathol. 141, 62–71 (2014).
Ravelli, A. et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J. Pediatr. 146, 598–604 (2005).
Minoia, F. et al. Development of new classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis [abstract]. Pediatr. Rheumatol. 12 (Suppl. 1) O1 (2014).
Reddy, V. V., Myles, A., Cheekatla, S. S., Singh, S. & Aggarwal, A. Soluble CD25 in serum: a potential marker for subclinical macrophage activation syndrome in patients with active systemic onset juvenile idiopathic arthritis. Int. J. Rheum. Dis. 17, 261–267 (2014).
Gorelik, M. et al. Follistatin-like protein 1 and the ferritin/erythrocyte sedimentation rate ratio are potential biomarkers for dysregulated gene expression and macrophage activation syndrome in systemic juvenile idiopathic arthritis. J. Rheumatol. 40, 1191–1199 (2013).
Shimizu, M., Nakagishi, Y. & Yachie, A. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine 61, 345–348 (2013).
Gracie, J. A., Robertson, S. E. & McInnes, I. B. Interleukin-18. J. Leukoc. Biol. 73, 213–224 (2003).
McInnes, I. B., Gracie, J. A., Leung, B. P., Wei, X. Q. & Liew, F. Y. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol. Today 21, 312–315 (2000).
de Jager, W. et al. Defective phosphorylation of interleukin-18 receptor β causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 60, 2782–2793 (2009).
Lattanzi, B. & Ravelli, A. in Textbook of Clinical Pediatrics (eds Elzouki, A. Y. et al.), 1629–1641 (Springer, 2012).
Weening, J. J. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 15, 241–250 (2004).
Rovin, B. H., Birmingham, D. J., Nagaraja, H. N., Yu, C. Y. & Hebert, L. A. Biomarker discovery in human SLE nephritis. Bull. NYU Hosp. Jt. Dis. 65, 187–193 (2007).
Batal, I. et al. Prospective assessment of C4d deposits on circulating cells and renal tissues in lupus nephritis: a pilot study. Lupus 21, 13–26 (2012).
Dhir, V. Is cellular C4d a good biomarker for SLE nephritis? Lupus 21, 1036 (2012).
Edelbauer, M. et al. Markers of childhood lupus nephritis indicating disease activity. Pediatr. Nephrol. 26, 401–410 (2011).
Hewitt, S. M., Dear, J. & Star, R. A. Discovery of protein biomarkers for renal diseases. J. Am. Soc. Nephrol. 15, 1677–1689 (2004).
Rovin, B. H. The chemokine network in systemic lupus erythematous nephritis. Front. Biosci. 13, 904–922 (2008).
Rovin, B. H. et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J. Am. Soc. Nephrol. 16, 467–473 (2005).
Kiani, A. N. et al. Urine osteoprotegerin and monocyte chemoattractant protein-1 in lupus nephritis. J. Rheumatol. 36, 2224–2230 (2009).
Tucci, M. et al. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 50, 1842–1849 (2004).
Watson, L. et al. Urinary monocyte chemoattractant protein 1 and α 1 acid glycoprotein as biomarkers of renal disease activity in juvenile-onset systemic lupus erythematosus. Lupus 21, 496–501 (2012).
Wu, T. et al. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J. Immunol. 179, 7166–7175 (2007).
Graves, D. T., Alsulaimani, F., Ding, Y. & Marks, S. C. Jr. Developmentally regulated monocyte recruitment and bone resorption are modulated by functional deletion of the monocytic chemoattractant protein-1 gene. Bone 31, 282–287 (2002).
Zhang, X. et al. Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int. 74, 799–807 (2008).
Tian, S. et al. Urinary levels of RANTES and M-CSF are predictors of lupus nephritis flare. Inflamm. Res. 56, 304–310 (2007).
Chan, R. W. et al. The effect of immunosuppressive therapy on the messenger RNA expression of target genes in the urinary sediment of patients with active lupus nephritis. Nephrol. Dial. Transplant. 21, 1534–1540 (2006).
Gwira, J. A. et al. Expression of neutrophil gelatinase-associated lipocalin regulates epithelial morphogenesis in vitro. J. Biol. Chem. 280, 7875–7882 (2005).
Brunner, H. I. et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 54, 2577–2584 (2006).
Watson, L. et al. Urine biomarkers for monitoring juvenile lupus nephritis: a prospective longitudinal study. Pediatr. Nephrol. 29, 397–405 (2014).
Hinze, C. H. et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 60, 2772–2781 (2009).
Suzuki, M. et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr. Nephrol. 23, 403–412 (2008).
Campbell, S., Michaelson, J., Burkly, L. & Putterman, C. The role of TWEAK/Fn14 in the pathogenesis of inflammation and systemic autoimmunity. Front. Biosci. 9, 2273–2284 (2004).
Campbell, S. et al. Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J. Immunol. 176, 1889–1898 (2006).
Schwartz, N. et al. Urinary TWEAK and the activity of lupus nephritis. J. Autoimmun. 27, 242–250 (2006).
Schwartz, N. et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicentre cohort study. Arthritis Res. Ther. 11, R143 (2009).
Abulaban, K. et al. A78: urine biomarkers role in predicting the future development of renal functional loss with lupus nephritis in children and adults. Arthritis Rheumatol. 66 (Suppl. 11), S111 (2014).
Avihingsanon, Y. et al. Measurement of urinary chemokine and growth factor messenger RNAs: a noninvasive monitoring in lupus nephritis. Kidney Int. 69, 747–753 (2006).
Wu, T. et al. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J. Immunol. 179, 7166–7175 (2007).
Wang, G. et al. Urinary FOXP3 mRNA in patients with lupus nephritis—relation with disease activity and treatment response. Rheumatology (Oxford) 48, 755–760 (2009).
Hammad, A. M., Youssef, H. M. & El-Arman, M. M. Transforming growth factor β 1 in children with systemic lupus erythematosus: a possible relation with clinical presentation of lupus nephritis. Lupus 15, 608–612 (2006).
Suzuki, M. et al. Identification of a urinary proteomic signature for lupus nephritis in children. Pediatr. Nephrol. 22, 2047–2057 (2007).
Suzuki, M. et al. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr. Res 65, 530–536 (2009).
Brunner, H. I. et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum. 64, 2687–2697 (2012).
Wedderburn, L. R. & Rider, L. G. Juvenile dermatomyositis: new developments in pathogenesis, assessment and treatment. Best. Pract. Res. Clin. Rheumatol. 23, 665–678 (2009).
Rider, L. G. & Miller, F. W. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA 305, 183–190 (2011).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292, 344–347 (1975).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 292, 403–407 (1975).
Brown, V. E., Pilkington, C. A., Feldman, B. M. & Davidson, J. E. An international consensus survey of the diagnostic criteria for juvenile dermatomyositis (JDM). Rheumatology (Oxford) 45, 990–993 (2006).
Davis, W. R. et al. Assessment of active inflammation in juvenile dermatomyositis: a novel magnetic resonance imaging-based scoring system. Rheumatology (Oxford) 50, 2237–2244 (2011).
Malattia, C. et al. Whole-body MRI in the assessment of disease activity in juvenile dermatomyositis. Ann. Rheum. Dis. 73, 1083–1090 (2014).
Gunawardena, H., Betteridge, Z. E. & McHugh, N. J. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford) 48, 607–612 (2009).
Tansley, S. L., McHugh, N. J. & Wedderburn, L. R. Adult and juvenile dermatomyositis: are the distinct clinical features explained by our current understanding of serological subgroups and pathogenic mechanisms? Arthritis Res. Ther. 15, 211 (2013).
Gunawardena, H. et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology (Oxford) 47, 324–328 (2008).
Rider, L. G. et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 92, 223–243 (2013).
Rider, L. G., Katz, J. D. & Jones, O. Y. Developments in the classification and treatment of the juvenile idiopathic inflammatory myopathies. Rheum. Dis. Clin. North Am. 39, 877–904 (2013).
Bingham, A. et al. Predictors of acquired lipodystrophy in juvenile-onset dermatomyositis and a gradient of severity. Medicine (Baltimore) 87, 70–86 (2008).
Espada, G., Maldonado Cocco, J. A., Fertig, N. & Oddis, C. V. Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J. Rheumatol. 36, 2547–2551 (2009).
Gunawardena, H. et al. Autoantibodies to a 140-kD protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum. 60, 1807–1814 (2009).
Huber, A. M. et al. Early illness features associated with mortality in the juvenile idiopathic inflammatory myopathies. Arthritis Care Res. (Hoboken) 66, 732–740 (2014).
Rouster-Stevens, K. A. & Pachman, L. M. Autoantibody to signal recognition particle in African American girls with juvenile polymyositis. J. Rheumatol. 35, 927–929 (2008).
Sato, S. et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 60, 2193–2200 (2009).
Kobayashi, I. et al. Anti-melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J. Pediatr. 158, 675–677 (2011).
Tansley, S. et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res. Ther. 16, R138 (2014).
Aggarwal, R. et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 66, 740–749 (2014).
Peixoto, D., Costa, J., Ferretti, M., Malattia, C. & Martini, A. New autoantibodies and their clinical associations in juvenile myositis—a systematic review. Acta Reumatol. Port. 38, 234–241 (2013).
Shah, M. et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 92, 25–41 (2013).
Wedderburn, L. R. et al. HLA class II haplotype and autoantibody associations in children with juvenile dermatomyositis and juvenile dermatomyositis-scleroderma overlap. Rheumatology (Oxford) 46, 1786–1791 (2007).
Author information
Authors and Affiliations
Contributions
A.R. and A.C. wrote the manuscript and contributed substantially to discussions of its content. All authors (A.R., A.C., G.C.V. and A.M.) researched data for the article and undertook review or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Consolaro, A., Varnier, G., Martini, A. et al. Advances in biomarkers for paediatric rheumatic diseases. Nat Rev Rheumatol 11, 265–275 (2015). https://doi.org/10.1038/nrrheum.2014.208
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.208
This article is cited by
-
Myeloid-related protein 8/14 in plasma and serum in patients with new-onset juvenile idiopathic arthritis in real-world setting in a single center
Pediatric Rheumatology (2022)
-
Rheumatology Panel in Pediatric Practice
Indian Pediatrics (2019)
-
Innovative Research Design to Meet the Challenges of Clinical Trials for Juvenile Dermatomyositis
Current Rheumatology Reports (2018)
-
Review of biomarkers in systemic juvenile idiopathic arthritis: helpful tools or just playing tricks?
Arthritis Research & Therapy (2016)
-
Juvenile Idiopathic Arthritis: Diagnosis and Treatment
Rheumatology and Therapy (2016)