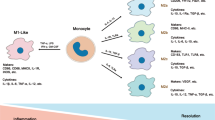
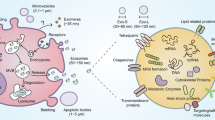
Key Points
-
The evolutionarily conserved release of extracellular vesicles (EVs), including exosomes, microvesicles (microparticles) and apoptotic vesicles, is a previously unappreciated type of secretion by cells
-
EVs trigger inflammation by carrying pathogen-associated and damage-associated molecular patterns or pathogenic autoantigens
-
EV-associated cytokines, lipid mediators and microRNAs contribute to the propagation phase of inflammatory diseases
-
EVs contain proteases and glycosidases that could contribute to tissue destruction
-
EVs could be used as novel therapeutic vehicles, immunomodulators and therapeutic targets
Abstract
The discovery that submicron-sized extracellular vesicles (EVs) are generated by both prokaryotic and eukaryotic cells might have a profound effect on experimental and clinical sciences, and could pave the way for new strategies to combat various diseases. EVs are carriers of pathogen-associated and damage-associated molecular patterns, cytokines, autoantigens and tissue-degrading enzymes. In addition to a possible role in the pathogenesis of a number of inflammatory conditions, such as infections and autoimmune diseases, EVs, including microvesicles (also known as microparticles), exosomes and apoptotic vesicles, have therapeutic potential and might be used as biomarkers for inflammatory diseases. Therefore, molecular diagnostics and targeted therapy could benefit from expanding knowledge in the field. In this Review, we summarize important developments and propose that extracellular vesicles could be used as therapeutic vehicles and as targets for the treatment and prevention of inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Théry, C., Ostrowski, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
György, B. et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68, 2667–2688 (2011).
van der Pol., E., Böing, A. N., Harrison, P., Sturk, A. & Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 64, 676–705 (2012).
Akers, J. C., Gonda, D., Kim, R., Carter, B. S. & Chen, C. C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 113, 1–11 (2013).
Morello, M. et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 12, 3526–3536 (2013).
Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 (2013).
Muralidharan-Chari, V., Clancy, J. W., Sedgwick, A. & D' Souza-Schorey, C. Microvesicles: mediators of extracellular communication during cancer progression. J. Cell Sci. 123, 1603–1611 (2010).
Nolte-'t Hoen, E. N., Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine 8, 712–720 (2012).
van der Pol., E., van Gemert, M. J., Sturk, A., Nieuwland, R. & van Leeuwen, T. G. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemost. 10, 919–930 (2012).
Lacroix, R., Robert, S., Poncelet, P., Kasthuri, R. S., Key, N. S. & Dignat-George, F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J. Thromb. Haemost. 8, 2571–2574 (2010).
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L. & Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420 (1987).
Verweij, F. J. et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 30, 2115–2129 (2011).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell. Biol. 9, 654–659 (2007).
Pilzer, D., Gasser, O., Moskovich, O., Schifferli, J. A. & Fishelson, Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin. Immunopathol. 27, 375–387 (2005).
Regev-Rudzki, N. et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 153, 1120–1133 (2013).
Timar, C. I. et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 121, 510–518 (2013).
Goh, F. G. & Midwood, K. S. Intrinsic danger: activation of Toll-like receptors in rheumatoid arthritis. Rheumatology (Oxford) 51, 7–23, (2012).
Deatherage, B. L. & Cookson, B. T. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 80, 1948–1957 (2012).
Nakao, R. et al. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS ONE 6, e26163 (2011).
Kaparakis, M. et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 12, 372–385 (2010).
Hong, S. W. et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 66, 351–359 (2011).
Kim, M. R. et al. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both TH1 and TH17 cell responses. Allergy 67, 1271–1281 (2012).
Prados-Rosales, R. et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 121, 1471–1483 (2011).
Gehrmann, U. et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses--novel mechanisms for host-microbe interactions in atopic eczema. PLoS ONE 6, e21480 (2011).
Schiller, M. et al. During apoptosis HMGB1 is translocated into apoptotic cell-derived membraneous vesicles. Autoimmunity 46, 342–346 (2013).
Ayna, G. et al. ATP release from dying autophagic cells and their phagocytosis are crucial for inflammasome activation in macrophages. PLoS ONE 7, e40069, (2012).
Turiak, L. et al. Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J. Proteomics 74, 2025–2033 (2011).
Cloutier, N. et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol. Med. 5, 235–249 (2013).
Skriner, K., Adolph, K., Jungblut, P. R. & Burmester, G. R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 54, 3809–3814 (2006).
Nielsen, C. T. et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 64, 1227–1236 (2012).
Pisetsky, D. S. Microparticles as autoantigens: making immune complexes big. Arthritis Rheum. 64, 958–961 (2012).
Ullal, A. J. et al. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J. Autoimmun. 36, 173–180 (2011).
Ullal, A. J. & Pisetsky, D. S. The role of microparticles in the generation of immune complexes in murine lupus. Clin. Immunol. 146, 1–9 (2013).
Kapsogeorgou, E. K., Abu-Helu, R. F., Moutsopoulos, H. M. & Manoussakis, M. N. Salivary gland epithelial cell exosomes: a source of autoantigenic ribonucleoproteins. Arthritis Rheum. 52, 1517–1521 (2005).
Mor-Vaknin, N. et al. DEK in the synovium of patients with juvenile idiopathic arthritis: characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum. 63, 556–567 (2011).
Silva, M. T. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 584, 4491–4499 (2010).
Lamkanfi, M. Emerging inflammasome effector mechanisms. Nat. Rev. Immunol. 11, 213–220 (2011).
Sheng, H. et al. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J. Immunol. 187, 1591–1600 (2011).
Rahman, M. J., Regn, D., Bashratyan, R. & Dai, Y. D. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes. http://dx.doi:10.2337/db13-0859.
Kojima, F., Kapoor, M., Kawai, S. & Crofford, L. J. New insights into eicosanoid biosynthetic pathways: implications for arthritis. Expert Rev. Clin. Immunol. 2, 277–291 (2006).
Barry, O. P., Pratico, D., Lawson, J. A. & FitzGerald, G. A. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Invest. 99, 2118–2127 (1997).
Esser, J. et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 126, 1032–1040 (2010).
Gulinelli, S. et al. IL-18 associates to microvesicles shed from human macrophages by a LPS/TLR-4 independent mechanism in response to P2X receptor stimulation. Eur. J. Immunol. 42, 3334–3345 (2012).
Nickel, W. & Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148–155 (2009).
Pizzirani, C. et al. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood 109, 3856–3864 (2007).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Baj-Krzyworzeka, M. et al. Tumour-derived microvesicles contain interleukin-8 and modulate production of chemokines by human monocytes. Anticancer Res. 31, 1329–1335 (2011).
Truman, L. A. et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood 112, 5026–5036 (2008).
Fabbri, M. TLRs as miRNA receptors. Cancer Res. 72, 6333–6337 (2012).
Ohshima, K. et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS ONE 5, e13247 (2010).
Lehmann, S. M. et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 15, 827–835 (2012).
Fabbri, M. et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl Acad. Sci. USA 109, E2110–E2116 (2012).
Fabbri, M., Paone, A., Calore, F., Galli, R. & Croce, C. M. A new role for microRNAs, as ligands of Toll-like receptors. RNA Biol. 10, 169–174 (2013).
Laffont, B. et al. Activated platelets can deliver mRNA regulatory Ago2–microRNA complexes to endothelial cells via microparticles. Blood 122, 253–261 (2013).
Lo Cicero, A., Majkowska, I., Nagase, H., Di Liegro, I. & Troeberg, L. Microvesicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol. 31, 229–233 (2012).
Shimoda, M. & Khokha, R. Proteolytic factors in exosomes. Proteomics 13, 1624–1636 (2013).
Li, C. J. et al. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am. J. Pathol. 182, 1552–1562 (2013).
Ortutay, Z. et al. Synovial fluid exoglycosidases are predictors of rheumatoid arthritis and are effective in cartilage glycosaminoglycan depletion. Arthritis Rheum. 48, 2163–2172 (2003).
Pasztoi, M. et al. Gene expression and activity of cartilage degrading glycosidases in human rheumatoid arthritis and osteoarthritis synovial fibroblasts. Arthritis Res. Ther. 11, R68 (2009).
Pasztoi, M. et al. The recently identified hexosaminidase D enzyme substantially contributes to the elevated hexosaminidase activity in rheumatoid arthritis. Immunol. Lett. 149, 71–76 (2013).
Knijff-Dutmer, E. A., Koerts, J., Nieuwland, R., Kalsbeek-Batenburg, E. M. & van de Laar, M. A. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 46, 1498–1503 (2002).
Berckmans, R. J. et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 46, 2857–2866 (2002).
Sellam, J. et al. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 11, R156 (2009).
György, B. et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE 7, e49726 (2012).
Wang, H. et al. Oxidized low-density lipoprotein-dependent platelet-derived microvesicles trigger procoagulant effects and amplify oxidative stress. Mol. Med. 18, 159–166 (2012).
Rautou, P. E. et al. Microparticles, vascular function, and atherothrombosis. Circ. Res. 109, 593–606 (2011).
Messer, L. et al. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res. Ther. 11, R40 (2009).
Berckmans, R. J. et al. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res. Ther. 7, R536–544 (2005).
Gyorgy, B. et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes due to shared biophysical parameters. Blood 117, e39–e48 (2011).
Jungel, A. et al. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 56, 3564–3574 (2007).
Distler, J. H. et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl Acad. Sci. USA 102, 2892–2897 (2005).
Reich, N. et al. Microparticles stimulate angiogenesis by inducing ELR(+) CXC-chemokines in synovial fibroblasts. J. Cell. Mol. Med. 15, 756–762 (2011).
Pereira, J. et al. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb. Haemost. 95, 94–99 (2006).
Ostergaard, O. et al. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. http://dx.doi.org/10.1002/art.38065.
Pisetsky, D. S., Gauley, J. & Ullal, A. J. Microparticles as a source of extracellular DNA. Immunol. Res. 49, 227–234 (2011).
Parker, B. et al. Suppression of inflammation reduces endothelial microparticles in active systemic lupus erythematosus. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-203028.
Guiducci, S. et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum. 58, 2845–2853 (2008).
Sgonc, R. et al. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J. Clin. Invest. 98, 785–792 (1996).
Aharon, A., Tamari, T. & Brenner, B. Monocyte derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost. 100, 878–885 (2008).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Cloutier, N. et al. Platelets can enhance vascular permeability. Blood 120, 1334–1343 (2012).
Sun, D. et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 18, 1606–1614 (2010).
Kooijmans, S. A. et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control Release 172, 229–238 (2013).
Bolukbasi, M. F. et al. miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol. Ther. Nucleic. Acids. 1, e10 (2012).
Shen, B., Wu, N., Yang, J. M. & Gould, S. J. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J. Biol. Chem. 286, 14383–14395 (2011).
Maguire, C. A. et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 20, 960–971 (2012).
Wahlgren, J. et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 40, e130 (2012).
Ohno, S. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21, 185–191 (2013).
Akao, Y. et al. Microvesicle-mediated RNA molecule delivery system using monocytes/macrophages. Mol. Ther. 19, 395–399 (2011).
Zhuang, X. et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19, 1769–1779 (2011).
Wang, G. J. et al. Thymus exosomes-like particles induce regulatory T cells. J. Immunol. 181, 5242–5248 (2008).
Yang, X., Meng, S., Jiang, H., Chen, T. & Wu, W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand. J. Gastroenterol. 45, 1168–1177 (2010).
Kim, S. H. et al. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 174, 6440–6448 (2005).
Ruffner, M. A. et al. B7–1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. Eur. J. Immunol. 39, 3084–3090 (2009).
Kim, S. H., Bianco, N. R., Shufesky, W. J., Morelli, A. E. & Robbins, P. D. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J. Immunol. 179, 2235–2241 (2007).
Cai, Z. et al. Immunosuppressive exosomes from TGF-beta1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 22, 607–610 (2012).
Kim, S. H., Bianco, N. R., Shufesky, W. J., Morelli, A. E. & Robbins, P. D. Effectivetreatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J. Immunol. 179, 2242–2249 (2007).
Kim, S. H. et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 13, 289–300 (2006).
Bianco, N. R., Kim, S. H., Ruffner, M. A. & Robbins, P. D. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 60, 380–389 (2009).
Zhang, J. et al. Circulating TNFR1 exosome-like vesicles partition with the LDL fraction of human plasma. Biochem. Biophys. Res. Commun. 366, 579–584 (2008).
Meijer, H., Reinecke, J., Becker, C., Tholen, G. & Wehling, P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm. Res. 52, 404–407 (2003).
Kelly, R. W. et al. Extracellular organelles (prostasomes) are immunosuppressive components of human semen. Clin. Exp. Immunol. 86, 550–556 (1991).
Shen, Y. et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509–520 (2012).
Savina, A., Furlan, M., Vidal, M. & Colombo, M. I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278, 20083–20090 (2003).
Thompson, C. A., Purushothaman, A., Ramani, V. C., Vlodavsky, I. & Sanderson, R. D. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 288, 10093–10099 (2013).
Blanchard, N. et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 168, 3235–3241 (2002).
Qu, Y. et al. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J. Immunol. 182, 5052–5062 (2009).
Constantinescu, P. et al. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim. Biophys. Acta 1798, 1797–1804 (2010).
Crespin, M., Vidal, C., Picard, F., Lacombe, C. & Fontenay, M. Activation of PAK1/2 during the shedding of platelet microvesicles. Blood Coagul. Fibrinolysis 20, 63–70 (2009).
Parolini, I. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 (2009).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Smith, S. K. et al. Mechanisms by which intracellular calcium induces susceptibility to secretory phospholipase A2 in human erythrocytes. J. Biol. Chem. 276, 22732–22741 (2001).
Barteneva, N. S. et al. Circulating microparticles: square the circle. BMC Cell Biol. 14, 23 (2013).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 (2010).
Muralidharan-Chari, V. et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875–1885 (2009).
Crescitelli, R. et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracellul. Vesicles, 2, 20677 (2013).
Acknowledgements
This work was supported by OTKA NK 84,043 (E.I.B.) and K77537 (G.N.), and FP7-PEOPLE-2011-ITN-PITN-GA-2011-289,033 “DYNANO” (E.I.B.). This research was supported by the European Union and the State of Hungary, cofinanced by the European Social Fund in the framework of TÁMOP 4.2.4. A/-11-1-2012-0001 'National Excellence Program' (B.G.).
Author information
Authors and Affiliations
Contributions
E.I.B. and B.G. researched the data for the article, substantially contributed to discussion of the article and wrote the article. E.I.B., G.N., A.F. and S.G. contributed to reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Buzas, E., György, B., Nagy, G. et al. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 10, 356–364 (2014). https://doi.org/10.1038/nrrheum.2014.19
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.19
This article is cited by
-
Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Asthma in Murine Models: A Systematic Review and Meta-analysis
Stem Cell Reviews and Reports (2024)
-
Low-intensity pulsed ultrasound (LIPUS) enhances the anti-inflammatory effects of bone marrow mesenchymal stem cells (BMSCs)-derived extracellular vesicles
Cellular & Molecular Biology Letters (2023)
-
Plasma gelsolin levels are associated with diabetes, sex, race, and poverty
Journal of Translational Medicine (2023)
-
Disease-microenvironment modulation by bare- or engineered-exosome for rheumatoid arthritis treatment
Biomaterials Research (2023)
-
Understanding the multifaceted role of miRNAs in Alzheimer’s disease pathology
Metabolic Brain Disease (2023)