Key Points
-
Paraneoplasias are caused by soluble factors produced by tumour cells or are the consequence of an immune reaction against these cells
-
An underlying malignancy should be sought if musculoskeletal symptoms do not show an adequate response to therapy
-
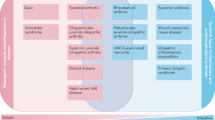
Arthritis, myositis, periostitis, fasciitis and osteomalacia can all be musculoskeletal manifestations of a paraneoplastic disease
-
Due to the properties of soluble factors produced by tumour cells, some paraneoplasias show a characteristic involvement of specific musculoskeletal tissues (e.g. VEGF inducing HOA or FGF23 inducing TIO)
-
Despite their rarity, knowledge of distinct clinical patterns of paraneoplasias is essential for the rheumatologist, because their recognition allows for a timely diagnosis and potentially life-saving therapy
-
The dissection of underlying molecular mechanisms can provide insights in the pathogenesis for rheumatic as well as neoplastic diseases
Abstract
For patients that present with musculoskeletal symptoms, diagnostic procedures carried out by physicians and rheumatologists are primarily aimed at confirming or excluding the occurrence of primary rheumatic diseases. Another important trigger for musculoskeletal disease, however, is the presence of a tumour. Careful clinical investigation and knowledge of the gestalt of musculoskeletal syndromes related to respective tumour entities is of utmost importance for the diagnosis of paraneoplastic rheumatic diseases such as hypertrophic osteoarthropathy, paraneoplastic polyarthritis, RS3PE syndrome, palmar fasciitis and polyarthritis, cancer-associated myositis and tumour-induced osteomalacia. This places great responsibility on rheumatologists in diagnosing malignancies and referring the patient for effective treatment. The selective influence of tumours on musculoskeletal tissue is surprising and indicates that tumours alter tissues such as the periosteum, synovial membrane, subcutaneous connective tissue, fascia, muscles and bones by specific molecular processes. Some of the underlying mechanisms have been unravelled, providing valuable information on the physiologic and pathophysiologic roles of mediators such as vascular endothelial growth factor and fibroblast growth factor 23.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Azar, L. & Khasnis, A. Paraneoplastic rheumatologic syndromes. Curr. Opin. Rheumatol. 25, 44–49 (2013).
Von Bamberger, E. Veränderungen der Röhrenknochen bei Bronchiektasie [German]. Wien. Klin. Wochenschr. 2, 226–240 (1889).
Marie, P. De l´ostéo-arthropathie hypertrophiante pneumonique [French]. Rev. Med. Paris 10, 1–36 (1890).
Craig, J. W. Hypertrophic pulmonary osteoarthropathy as the first symptom of pulmonary neoplasm. Brit. Med. J. 1, 750–752 (1937).
Vogl, A., Blumenfeld, S. & Gutner, L. B. Diagnostic significance of pulmonary hypertrophic osteoarthropathy. Am. J. Med. 18, 51–65 (1955).
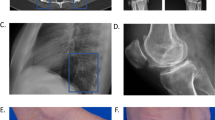
Pineda, C. & Martínez-Lavín, M. Hypertrophic osteoarthropathy: what a rheumatologist should know about this uncommon condition. Rheum. Dis. Clin. North Am. 39, 383–400 (2013).
Manger, B. et al. Clinical Images: Hippokrates confirmed by positron emission tomography. Arthritis Rheum. 63, 1150 (2011).
Martínez-Lavín, M. Exploring the cause of the most ancient clinical sign in medicine: finger clubbing. Semin. Arthritis Rheum. 36, 380–385 (2007).
Rutherford, J. D. Digital clubbing. Circulation 127, 1997–1999 (2013).
Izumi, M., Takayama, K., Yabuuchi, H., Abe, K. & Nakanishi, Y. Incidence of hypertrophic pulmonary osteoarthropathy associated with primary lung cancer. Respirology 15, 809–812 (2010).
Ito, T. et al. Hypertrophic pulmonary osteoarthropathy as a paraneoplastic manifestation of lung cancer. J. Thorac. Oncol. 5, 976–980 (2010).
Cohen, P. R. Hypertrophic pulmonary osteoarthropathy and tripe palms in a man with squamous cell carcinoma of the larynx and lung. Am. J. Clin. Oncol. 16, 268–276 (1993).
Saeed, H. & Massarweh, S. Images in clinical medicine. Hypertrophic pulmonary osteoarthropathy and tripe palms. N. Engl. J. Med. 366, 360 (2012).
Spicknall, K. E., Zirwas, M. J. & English III, J. C. Clubbing: An update on diagnosis, pathophysiology, and clinical relevance. J. Am. Acad. Dermatol. 52, 1020–1028 (2005).
Gosney, M. A., Gosney, J. R. & Lye, M. Plasma growth hormone and digital clubbing in carcinoma of the bronchus. Thorax 45, 545–547 (1990).
Yorgancioğlu, A., Akin, M., Demtray, M. & Derelt, S. The relationship between digital clubbing and serum growth hormone level in patients with lung cancer. Monaldi Arch. Chest Dis. 51, 185–187 (1996).
Martínez-Lavín, M. Digital clubbing and hypertrophic osteoarthropathy: a unifying hypothesis. J. Rheumatol. 14, 6–8 (1987).
Dickinson, C. J. & Martin, J. F. Megakaryocytes and platelet clumps as the cause of finger clubbing. Lancet 2, 1434–1435 (1987).
Fox, S. B., Day, C. A. & Gatter, K. C. Association between platelet microthrombi and finger clubbing. Lancet 338, 313–314 (1991).
Matucci-Cerinic, M., Martinez-Lavin, M., Rojo, F., Fonseca, C. & Kahaleh, B. M. Von Willebrand factor antigen in hypertrophic osteoarthropathy. J. Rheumatol. 19, 765–767 (1992).
Silveri, E. et al. Hypertrophic osteoarthropathy: endothelium and platelet function. Clin. Rheumatol. 15, 435–439 (1996).
Atkinson, S. & Fox, S. B. Vascular endothelial growth factor (VEGF)-A and platelet-derived growth factor (PDGF) play a central role in the pathogenesis of digital clubbing. J. Pathol. 203, 721–728 (2004).
Silveira, L. H. et al. Vascular endothelial growth factor and hypertrophic osteoarthropathy. Clin. Exp. Rheumatol. 18, 57–62 (2000).
Abe, Y. et al. A case of pulmonary adenocarcinoma associated with hypertrophic osteoarthropathy due to vascular endothelial growth factor. Anticancer Res. 22, 3485–3488 (2002).
Olán, F. et al. Circulating vascular endothelial growth factor concentrations in a case of pulmonary hypertrophic osteoarthropathy. J. Rheumatol. 31, 614–616 (2004).
Hah, Y. S. et al. Vascular endothelial growth factor stimulates osteoblastic differentiation of cultured human periosteal-derived cells expressing vascular endothelial growth factor receptors. Mol. Biol. Rep. 38, 1443–1450 (2011).
Uppal, S. et al. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat. Genet. 40, 789–793 (2008).
Zhang, Z., He, J. W., Fu, W. Z., Zhang, C. Q. & Zhang, Z. L. A novel mutation in the SLCO2A1 gene in a Chinese family with primary hypertrophic osteoarthropathy. Gene 521, 191–194 (2013).
Harada, S. et al. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J. Clin. Invest. 93, 2490–2496 (1994).
Shih, W. J. Pulmonary hypertrophic osteoarthropathy and its resolution. Semin. Nucl. Med. 34, 159–163 (2004).
Ulusakarya, A. et al. Symptoms in cancer patients and an unusual tumor: Case 1. Regression of hypertrophic pulmonary osteoarthropathy following chemotherapy for lung metastases of a nasopharyngeal carcinoma. J. Clin. Oncol. 23, 9422–9423 (2005).
Kozak, K. R., Milne, G. L., Morrow, J. D. & Cuiffo, B. P. Hypertrophic osteoarthropathy pathogenesis: a case highlighting the potential role for cyclo-oxygenase-2-derived prostaglandin E2 . Nat. Clin. Pract. Rheumatol. 2, 452–456 (2006).
Johnson, S. A., Spiller, P. A. & Faull, C. M. Treatment of resistant pain in hypertrophic pulmonary osteoarthropathy with subcutaneous octreotide. Thorax 52, 298–299 (1997).
Angel-Moreno Maroto, A., Martínez-Quintana, E., Suárez-Castellano, L. & Pérez-Arellano, J. L. Painful hypertrophic osteoarthropathy successfully treated with octreotide. The pathogenetic role of vascular endothelial growth factor (VEGF). Rheumatology 44, 1326–1327 (2005).
Jayakar, B. A., Abelson, A. G. & Yao, Q. Treatment of hypertrophic osteoarthropathy with zoledronic acid: case report and review of the literature. Semin. Arthritis Rheum. 41, 291–296 (2011).
Santini, D. et al. Changes in bone resorption and vascular endothelial growth factor after a single zoledronic acid infusion in cancer patients with bone metastases from solid tumours. Oncol. Rep. 15, 1351–1357 (2006).
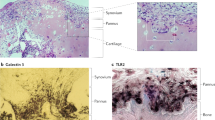
Naschitz, J. E. & Rosner, I. Musculoskeletal syndromes associated with malignancy (excluding hypertrophic osteoarthropathy). Curr. Opin. Rheumatol. 20, 100–105 (2008).
Pines, A., Kaplinsky, N., Olchovsky, D. & Frankl, O. Rheumatoid arthritis-like syndrome: A presenting symptom of malignancy. Report of 3 cases and review of the literature. Eur. J. Rheum. Inflam. 7, 51–55 (1984).
Alvarez Lario, B. et al. Poliartritis paraneoplásica. Descripción de cinco casos [Spanish]. Med. Clin. (Barc.) 88, 55–58 (1987).
Pfitzenmeyer, P., Bielefeld, P., Tavernier, C., Besancenot, J. F. & Gaudet, M. Aspects actuels de la polyarthrite aiguë paranéoplasique [French]. Rev. Med. Interne 13, 195–199 (1992).
Stummvoll, G. H., Aringer, M. Machold, K. P., Smolen, J. S. & Raderer, M. Cancer polyarthritis resembling rheumatoid arthritis as a first sign of hidden neoplasms. Scand. J. Rheumatol. 30, 40–44 (2001).
Morel, J. et al. Characteristics and survival of 26 patients with paraneoplastic arthritis. Ann. Rheum. Dis. 67, 244–247 (2008).
Hakkou, J., Rostom, S., Bahiri, R. & Hajjaj-Hassouni, N. Paraneoplastic syndromes: report of eight cases and review of literature. Rheumatol. Int. 32, 1485–1489 (2012).
Yamashita, H. et al. Characteristics of 10 patients with paraneoplastic rheumatologic musculoskeletal manifestations. Mod. Rheumatol. 24, 492–498 (2014).
Kisacik, B. et al. Diagnostic dilemma of paraneoplastic arthritis: case series. Int. J. Rheum. Dis. http://dx.doi.org/10.1111/1756-185X.12277.
Rugienė, R. et al. Prevalence of paraneoplastic rheumatic syndromes and their antibody profile among patients with solid tumours. Clin. Rheumatol. 30, 373–380 (2011).
Kumar, S., Sethi, S., Irani, F. & Bode, B. Y. Anticyclic citrullinated peptide antibody-positive paraneoplastic polyarthritis in a patient with metastatic pancreatic cancer. Am. J. Med. Sci. 338, 511–512 (2009).
Larson, E., Etwaru, D., Siva, C. & Lawlor, K. Report of anti-CCP antibody positive paraneoplastic polyarthritis and review of the literature. Rheumatol. Int. 31, 1635–1638 (2011).
Bradley, J. D. & Pinals, R. S. Carcinoma polyarthritis: role of immune complexes in pathogenesis. J. Rheumatol. 10, 826–828 (1983).
Schultz, H., Krenn, V. & Tony, H. P. Oligoarthritis mediated by tumor-specific T lymphocytes in renal-cell carcinoma. N. Engl. J. Med. 341, 290–291 (1999).
McCarty, D. J., O'Duffy, J. D., Pearson, L. & Hunter, J. B. Remitting seronegative symmetrical synovitis with pitting edema. RS3PE syndrome. JAMA 254, 2763–2767 (1985).
Yao, Q., Su, X. & Altman, R. D. Is remitting seronegative symmetrical synovitis with pitting edema (RS3PE) a subset of rheumatoid arthritis? Semin. Arthritis Rheum. 40, 89–94 (2010).
Sibilia, J. et al. Remitting seronegative symmetrical synovitis with pitting edema (RS3PE): a form of paraneoplastic polyarthritis? J. Rheumatol. 26, 115–120 (1999).
Paira, S., Graf, C., Roverano, S. & Rossini, J. Remitting seronegative symmetrical synovitis with pitting oedema: a study of 12 cases. Clin. Rheumatol. 21, 146–149 (2002).
Russell, E. B. Remitting seronegative symmetrical synovitis with pitting edema syndrome: followup for neoplasia. J. Rheumatol. 32, 1760–1761 (2005).
Fietta, P. & Manganelli, P. Remitting seronegative symmetrical synovitis with pitting edema syndrome: followup for neoplasia. J. Rheumatol. 33, 2365–2366 (2006).
Bucaloiu, I. D., Olenginski, T. P. & Harrington, T. M. Remitting seronegative symmetrical synovitis with pitting edema syndrome in a rural tertiary care practice: a retrospective analysis. Mayo Clin. Proc. 82, 1510–1515 (2007).
Origuchi, T. et al. High serum matrix metalloproteinase 3 is characteristic of patients with paraneoplastic remitting seronegative symmetrical synovitis with pitting edema syndrome. Mod. Rheumatol. 22, 584–588 (2012).
Arima, K. et al. RS3PE syndrome presenting as vascular endothelial growth factor associated disorder. Ann. Rheum. Dis. 64, 1653–1655 (2005).
Matsuda, M. et al. Sarcoidosis with high serum levels of vascular endothelial growth factor (VEGF), showing RS3PE-like symptoms in extremities. Clin. Rheumatol. 23, 246–248 (2004).
Tabeya, T. et al. A case of angioimmunoblastic T-cell lymphoma with high serum VEGF preceded by RS3PE syndrome. Mod. Rheumatol. http://dx.doi.org/10.3109/14397595.2013.857836.
Zucker, S, Vacirca, J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 23, 101–117 (2004).
Murphy, G. & Nagase, H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat. Clin. Pract. Rheumatol. 4, 128–135 (2007).
Roldan, M. R., Martinez, F., Roman, J. & Torres, A. Non-Hodgkin's lymphoma: initial manifestation. Ann. Rheum. Dis. 52, 85–86 (1993).
Chiappetta, N. & Gruber, B. Remitting seronegative symmetrical synovitis with pitting edema associated with acute myeloid leukemia. J. Rheumatol. 32, 1613–1614 (2004).
Tada, Y. et al. Remitting seronegative symmetrical synovitis with pitting edema associated with gastric carcinoma. J. Rheumatol. 24, 974–975 (1997).
Olivo, D. & Mattace, R. Concurrence of benign edematous polysynovitis in the elderly (RS3PE syndrome) and endometrial adenocarcinoma. Scand. J. Rheumatol. 26, 67–68 (1997).
Medsger, T. A., Dixon, J. A. & Garwood, V. F. Palmar fasciitis and polyarthritis associated with ovarian carcinoma. Ann. Intern. Med. 96, 424–431 (1982).
Bremer, C. Shoulder-hand syndrome. A case of unusual aetiology. Ann. Phys. Med. 9, 168–171 (1967).
Manger, B. & Schett, G. Palmar fasciitis and polyarthritis syndrome—systematic literature review of 100 cases. Semin. Arthritis Rheum. http://dx.doi.org/10.1016/j.semarthrit.2014.03.005.
Cox, N. H., Ramsay, B., Dobson, C. & Comaish, J. S. Woody hands in a patient with pancreatic carcinoma: a variant of cancer-associated fasciitis-panniculitis syndrome. Br. J. Dermatol. 135, 995–998 (1996).
Alexandroff, A. B. et al. Woody hands. Lancet 361, 1344 (2003).
Yogarajah, M., Soh, J., Lord, B., Goddard, N. & Stratton, R. Palmar fasciitis and polyarthritis syndrome: a sign of ovarian malignancy. J. R. Soc. Med. 101, 473–475 (2008).
Virik, K., Lynch, K. P. & Harper, P. Gastroesophageal cancer, palmar fasciitis and a matrix metalloproteinase inhibitor. Intern. Med. J. 32, 50–51 (2002).
Shiel, W. C. Jr., Prete, P. E., Jason, M. & Andrews, B. S. Palmar fasciitis and arthritis with ovarian and non-ovarian carcinomas. New syndrome. Am. J. Med. 79, 640–644 (1985).
Valverde-Garcia, J. et al. Paraneoplastic palmar fasciitis-polyarthritis syndrome associated with breast cancer. J. Rheumatol. 14, 1207–1209 (1987).
Goldberg, E., Dobransky, R. & Gill, R. Reflex sympathetic dystrophy associated with malignancy. Arthritis Rheum. 28, 1079–1080 (1985).
Enomoto, M. et al. Palmar fasciitis and polyarthritis associated with gastric carcinoma: complete resolution after total gastrectomy. Intern. Med. 39, 754–757 (2000).
Stertz, G. Polymyositis [German]. Berl. Klin. Wochenschr. 53, 489 (1916).
Zahr, Z. A. & Baer, A. N. Malignancy in myositis. Curr. Rheumatol. Rep. 13, 208–215 (2011).
Airio, A., Pukkala, E. & Isomäki, H. Elevated cancer incidence in patients with dermatomyositis: a population based study. J. Rheumatol. 22, 1300–1303 (1995).
Limaye, V. et al. The incidence and associations of malignancy in a large cohort of patients with biopsy-determined idiopathic inflammatory myositis. Rheumatol. Int. 33, 965–971 (2013).
Whitmore, S. E., Watson, R., Rosenshein, N. B. & Provost, T. T. Dermatomyositis sine myositis: association with malignancy. J. Rheumatol. 23, 101–105 (1996).
Hill, C. L. et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 357, 96–100 (2001).
Ang, P., Sugeng, M. W. & Chua, S. H. Classical and amyopathic dermatomyositis seen at the National Skin Centre of Singapore: a 3-year retrospective review of their clinical characteristics and association with malignancy. Ann. Acad. Med. Singapore 29, 219–223 (2000).
András, C. et al. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J. Rheumatol. 35, 438–444 (2008).
Chinoy, H., Fertig, N., Oddis, C. V., Ollier, W. E. & Cooper, R. G. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann. Rheum. Dis. 66, 1345–1349 (2007).
Casciola-Rosen, L. et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J. Exp. Med. 201, 591–601 (2005).
Targoff, I. N. et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 54, 3682–3689 (2006).
Zampieri, S. et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun. Rev. 9, 449–453 (2010).
Fujimoto, M. et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 64, 513–522 (2012).
Trallero-Araguás, E. et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 64, 523–532 (2012).
Allton, K. et al. Trim24 targets endogenous p53 for degradation. Proc. Natl Acad. Sci. USA 106, 11612–11616 (2009).
Labrador-Horrillo, M. et al. Anti-TIF1γ antibodies (anti-p155) in adult patients with dermatomyositis: comparison of different diagnostic assays. Ann. Rheum. Dis. 71, 993–996 (2012).
Ichimura, Y. et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann. Rheum. Dis. 71, 710–713 (2012).
McCance, R. A. Osteomalacia with Looser's nodes (Milkman's syndrome) due to a raised resistance to vitamin D acquired about the age of 15 years. Q. J. Med. 16, 33–46 (1947).
Chong, W. H., Molinolo, A. A. Chen, C. C. & Collins, M. T. Tumor-induced osteomalacia. Endocr. Relat. Cancer 18, R53–R77 (2011).
Prader, A., Illig, R., Uehlinger, E. & Stalder, G. Rickets following bone tumor [German]. Helv. Paediatr. Acta 14, 554–565 (1959).
White, K. E. et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J. Clin. Endocrinol. Metab. 86, 497–500 (2001).
Folpe, A. L. et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am. J. Surg. Pathol. 28, 1–30 (2004).
Jiang, Y. et al. Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: Report of 39 cases and review of the literature. J. Bone Miner. Res. 27, 1967–1975 (2012).
Hill, A. B. The environment and disease: association or causation? Proc. R. Soc. Med. 58, 293–300 (1965).
Author information
Authors and Affiliations
Contributions
B.M. researched the data for the article. B.M. and G.S. contributed substantially to discussions of the article content, writing the article and reviewing and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Manger, B., Schett, G. Paraneoplastic syndromes in rheumatology. Nat Rev Rheumatol 10, 662–670 (2014). https://doi.org/10.1038/nrrheum.2014.138
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.138
This article is cited by
-
Cardiac sarcoma presenting as paraneoplastic arthritis and clubbing: a case report and literature review
BMC Rheumatology (2023)
-
Paraneoplastische Syndrome in der Rheumatologie
Zeitschrift für Rheumatologie (2023)
-
Paraneoplastic musculoskeletal disorders: review and update for radiologists
Skeletal Radiology (2023)
-
bFGF could be a biomarker of malignancy in RS3PE syndrome: an ambispective single-center cohort analysis of 51 patients
Arthritis Research & Therapy (2021)
-
Pathogenesis, Diagnosis and Management of Polymyalgia Rheumatica
Drugs & Aging (2019)