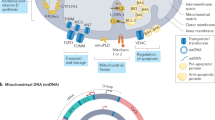
Key Points
-
Oxidative stress—generated through multiple mechanisms in a cell-type-specific manner—is a substantial contributor to disease pathogenesis, organ damage and comorbidities in patients with systemic lupus erythematosus (SLE)
-
Pathways of oxidative pathogenesis, such as oxidative modification of self antigens and T-cell dysfunction, have been identified
-
Organ systems in which the clinical importance of oxidative damage in SLE has been recognized include the cardiovascular and renal systems and the skin
-
Biomarkers of oxidative stress correlate directly with disease activity in SLE
-
Depletion of glutathione (reflecting oxidative stress) might have a pathogenic role; its reversal by N-acetylcysteine seems to have therapeutic benefit in mouse models and patients with SLE
Abstract
Oxidative stress is increased in systemic lupus erythematosus (SLE), and it contributes to immune system dysregulation, abnormal activation and processing of cell-death signals, autoantibody production and fatal comorbidities. Mitochondrial dysfunction in T cells promotes the release of highly diffusible inflammatory lipid hydroperoxides, which spread oxidative stress to other intracellular organelles and through the bloodstream. Oxidative modification of self antigens triggers autoimmunity, and the degree of such modification of serum proteins shows striking correlation with disease activity and organ damage in SLE. In T cells from patients with SLE and animal models of the disease, glutathione, the main intracellular antioxidant, is depleted and serine/threonine-protein kinase mTOR undergoes redox-dependent activation. In turn, reversal of glutathione depletion by application of its amino acid precursor, N-acetylcysteine, improves disease activity in lupus-prone mice; pilot studies in patients with SLE have yielded positive results that warrant further research. Blocking mTOR activation in T cells could conceivably provide a well-tolerated and inexpensive alternative approach to B-cell blockade and traditional immunosuppressive treatments. Nevertheless, compartmentalized oxidative stress in self-reactive T cells, B cells and phagocytic cells might serve to limit autoimmunity and its inhibition could be detrimental. Antioxidant therapy might also be useful in ameliorating damage caused by other treatments. This Review thus seeks to critically evaluate the complexity of oxidative stress and its relevance to the pathogenesis and treatment of SLE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Francis, L. & Perl, A. Pharmacotherapy of systemic lupus erythematosus. Expert Opin. Pharmacother. 10, 1481–1494 (2009).
Tsokos, G. C. Systemic Lupus Erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Perl, A. Systems biology of lupus: Mapping the impact of genomic and environmental factors on gene expression signatures, cellular signaling, metabolic pathways, hormonal and cytokine imbalance, and selecting targets for treatment. Autoimmunity 43, 32–47 (2010).
Perl, A., Hanczko, R., Telarico, T., Oaks, Z. & Landas, S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 7, 395–403 (2011).
Perl, A., Gergely, P. Jr, Nagy, G., Koncz, A. & Banki, K. Mitochondrial hyperpolarization: a checkpoint of T cell life, death, and autoimmunity. Trends Immunol. 25, 360–367 (2004).
Gergely, P. J. et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 46, 175–190 (2002).
Lai, Z.-W. et al. N-acetylcysteine reduces disease activity by blocking mTOR in T cells of lupus patients. Arthritis Rheum. 64, 2937–2946 (2012).
Gergely, P. J. et al. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J. Immunol. 169, 1092–1101 (2002).
Shah, D., Aggarwal, A., Bhatnagar, A., Kiran, R. & Wanchu, A. Association between T lymphocyte sub-sets apoptosis and peripheral blood mononuclear cells oxidative stress in systemic lupus erythematosus. Free Rad. Res. 45, 559–567 (2011).
Fernandez, D. R. et al. Activation of mTOR controls the loss of TCR in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J. Immunol. 182, 2063–2073 (2009).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Jackson, S. H., Devadas, S., Kwon, J., Pinto, L. A. & Williams, M. S. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 5, 818–827 (2004).
Segal, B. H., Grimm, M. J., Khan, A. N., Han, W. & Blackwell, T. S. Regulation of innate immunity by NADPH oxidase. Free Rad. Biol. Med. 53, 72–80 (2012).
Cooper, G. S., Makris, S. L., Nietert, P. J. & Jinot, J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ. Health Persp. 117, 696–702 (2009).
Perry, D. J. et al. Murine lupus susceptibility locus Sle1c2 mediates CD4+ T cell activation and maps to estrogen-related receptor γ. J. Immunol. 189, 793–803 (2012).
Yu, X. et al. Association of UCP2 -866 G/A polymorphism with chronic inflammatory diseases. Genes Immun. 10, 601–605 (2009).
Vyshkina, T. et al. Association of common mitochondrial DNA variants with multiple sclerosis and systemic lupus erythematosus. Clin. Immunol. 129, 31–35 (2008).
Yoh, K. et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 60, 1343–1353 (2001).
Fraser, P. A. et al. Glutathione S-transferase M null homozygosity and risk of systemic lupus erythematosus associated with sun exposure: a possible gene-environment interaction for autoimmunity. J. Rheumatol. 30, 276–282 (2003).
Lee, R., Margaritis, M., Channon, K. M. & Antoniades, C. Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr. Med. Chem. 19, 2504–2520 (2012).
Trager, J. & Ward, M. M. Mortality and causes of death in systemic lupus erythematosus. Curr. Opin. Rheumatol. 13, 345–351 (2001).
Tepel, M., van der Giet, M., Statz, M., Jankowski, J. & Zidek, W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure. Circulation 107, 992–995 (2003).
Demedts, M. et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 353, 2229–2242 (2005).
Skulachev, V. P. Mitochondrial physiology and pathology; concepts of programmed death of organelles, cells and organisms. Mol. Asp. Med. 20, 139–140 (1999).
Halliwell, B. & Gutteridge, J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Meth. Enzymol. 186, 1–85 (1990).
Cui, K., Luo, X., Xu, K. & Ven Murthy, M. R. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog. Neuropsychopharmacol Biol. Psychiatry 28, 771–799 (2004).
Nagy, G., Koncz, A. & Perl, A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J. Immunol. 171, 5188–5197 (2003).
Caza, T. N. et al. HRES-1/RAB4-mediated depletion of DRP1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann. Rheum. Dis. http://ard.bmj.com/content/early/2013/07/29/annrheumdis-2013-203794.
Caza, T. N., Talaber, G. & Perl, A. Metabolic regulation of organelle homeostasis in lupus T cells. Clin. Immunol. 144, 200–213 (2012).
Crabtree, M. J., Brixey, R., Batchelor, H., Hale, A. B. & Channon, K. M. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J. Biol. Chem. 288, 561–569 (2013).
Chen, C. A. et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468, 1115–1118 (2010).
Cordova, E. J., Velazquez-Cruz, R., Centeno, F., Baca, V. & Orozco, L. The NRF2 gene variant, –653G/A, is associated with nephritis in childhood-onset systemic lupus erythematosus. Lupus 19, 1237–1242 (2010).
Tsai, P. Y. et al. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum. 64, 232–242 (2012).
Lai, Z. et al. mTOR activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J. Immunol. 35, 2236–2246 (2013).
Doyle, H. A. & Mamula, M. J. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr. Opin. Immunol. 24, 112–118 (2012).
Casciola-Rosen, L., Andrade, F., Ulanet, D., Wong, W. B. & Rosen, A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J. Exp. Med. 190, 815–826 (1999).
Fraternale, A. et al. GSH and analogs in antiviral therapy. Mol. Aspects Med. 30, 99–110 (2009).
James, J. J. et al. An increased prevalence of Epstein–Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J. Clin. Invest. 100, 3019–3026 (1997).
Lunec, J., Herbert, K., Blount, S., Griffiths, H. R. & Emery, P. 8-Hydroxydeoxyguanosine. A marker of oxidative DNA damage in systemic lupus erythematosus. FEBS Lett. 348, 131–138 (1994).
Sauter, B. et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 (2000).
Ma, L. et al. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur. J. Immunol. 35, 3364–3375 (2005).
Perl, A., Fernandez, D., Telarico, T. & Phillips, P. E. Endogenous retroviral pathogenesis in lupus. Curr. Opin. Rheumatol. 22, 483–492 (2010).
Yang, Y. G., Lindahl, T. & Barnes, D. E. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131, 873–886 (2007).
Nagy, G. et al. Regulation of CD4 expression via recycling by HRES-1/RAB4 controls susceptibility to HIV infection. J. Biol. Chem. 281, 34574–34591 (2006).
Yan, N., Regalado-Magdos, A. D., Stiggelbout, B., Lee-Kirsch, M. A. & Lieberman, J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 11, 1005–1013 (2010).
Kuraoka, I. et al. Oxygen free radical damage to DNA: Translesion synthesis by human DNA polymerase eta and resistance to exonuclease action at cyclopurine deoxynucleoside residues. J. Biol. Chem. 276, 49283–49288 (2001).
Pisetsky, D. S. The origin and properties of extracellular DNA: From PAMP to DAMP. Clin. Immunol. 144, 32–40 (2012).
Kaplan, M. Neutrophils in the pathogenesis and manifestations of SLE. Nat. Rev. Rheumatol. 691–699 (2011).
Kurien, B. T., Hensley, K., Bachmann, M. & Scofield, R. H. Oxidatively modified autoantigens in autoimmune diseases. Free Radic. Biol. Med. 41, 549–556 (2006).
Villanueva, E. et al. netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Winkelstein, J. A. et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (Baltimore) 79, 155–169 (2000).
Campbell, A. M., Kashgarian, M. & Shlomchik, M. J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 4, 157ra141 (2012).
Tang, F. Y., Xie, X. W., Ling, G. H. & Liu, F. Y. Endothelial nitric oxide synthase and nicotinamide adenosine dinucleotide phosphate oxidase p22phox gene (C242T) polymorphisms and systemic lupus erythematosus in a Chinese Population. Lupus 19, 192–196 (2010).
Yu, B. et al. The association between single-nucleotide polymorphisms of NCF2 and systemic lupus erythematosus in Chinese mainland population. Clin. Rheumatol. 30, 521–527 (2011).
Jacob, C. O. et al. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc. Natl Acad. Sci. USA 109, E59–E67 (2012).
Cyr, A. R. & Domann, F. E. The redox basis of epigenetic modifications: From mechanisms to functional consequences. Antiox. Redox Signal. 15, 551–589 (2011).
Tang, H. et al. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 26, 4710–4721 (2012).
Gerashchenko, M. V., Lobanov, A. V. & Gladyshev, V. N. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc. Natl Acad. Sci. USA. 109, 17394–17399 (2012).
Margittai, E. & Sitia, R. Oxidative protein folding in the secretory pathway and redox signaling across compartments and cells. Traffic 12, 1–8 (2011).
Caza, T. N., Talaber, G. & Perl, A. Metabolic regulation of organelle homeostasis in lupus T cells. Clin. Immunol. 144, 200–213 (2012).
Shatynski, K. E., Chen, H., Kwon, J. & Williams, M. S. Decreased STAT5 phosphorylation and GATA-3 expression in NOX2-deficient T cells: Role in T helper development. Eur. J. Immunol. 42, 3202–3211 (2012).
Sieling, P. A. et al. CD1c-reactive, TH2 cytokine producing T-cells in human autoimmune disease. FASEB J. 12, A1091 (1998).
Tsokos, G. C. et al. Deficient gamma-interferon production in patients with systemic lupus erythematosus. Arthritis Rheum. 29, 1210–1215 (1986).
Sieling, P. A. et al. human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J. Immunol. 165, 5338–5344 (2000).
Dean, G. S., Anand, A., Blofeld, A., Isenberg, D. A. & Lydyard, P. M. Characterization of CD3+CD4−CD8− (double negative) T cells in patients with systemic lupus erythematosus: Production of IL-4. Lupus 11, 501–507 (2002).
Georgescu, L., Vakkalanka, R. K., Elkon, K. B. & Crow, M. K. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J. Clin. Invest. 100, 2622–2633 (1997).
Shivakumar, S., Tsokos, G. C. & Datta, S. K. T cell receptor α/β expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J. Immunol. 143, 103–112 (1989).
Wu, Z., MacPhee, I. A. M. & Oliveira, D. B. G. Reactive oxygen species in the initiation of IL-4 driven autoimmunity as a potential therapeutic target. Curr. Pharm. Design 10, 899–913 (2004).
Nagy, G., Barcza, M., Gonchoroff, N., Phillips, P. E. & Perl, A. Nitric oxide-dependent mitochondrial biogenesis generates Ca2+ signaling profile of lupus T cells. J. Immunol. 173, 3676–3683 (2004).
Richardson, B. et al. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 33, 1665–1673 (1990).
Sawalha, A. H. et al. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes Immun. 9, 368–378 (2008).
Gorelik, G. J., Yarlagadda, S. & Richardson, B. C. Protein kinase Cδ oxidation contributes to ERK inactivation in lupus T cells. Arthritis Rheum. 64, 2964–2974 (2012).
Anstee, Q. M. & Day, C. P. S-adenosylmethionine (SAMe) therapy in liver disease: A review of current evidence and clinical utility. J. Hepatol. 57, 1097–1109 (2012).
Imagawa, K. et al. The epigenetic effect of glucosamine and a nuclear factor-κ B (NF-κB) inhibitor on primary human chondrocytes—Implications for osteoarthritis. Biochem. Biophys. Res. Commun. 405, 362–367 (2011).
Tomasoni, R. et al. Rapamycin-sensitive signals control TCR/CD28-driven Ifng, Il4 and Foxp3 transcription and promoter region methylation. Eur. J. Immunol. 41, 2086–2096 (2011).
Yao, H. & Rahman, I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem. Pharmacol. 84, 1332–1339 (2012).
Mishra, N., Reilly, C. M., Brown, D. R., Ruiz, P. & Gilkeson, G. S. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J. Clin. Invest. 111, 539–552 (2003).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2012).
Myzak, M. C., Karplus, P. A., Chung, F. L. & Dashwood, R. H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 64, 5767–5774 (2004).
Schulze-Luehrmann, J. & Ghosh, S. Antigen-receptor signaling to nuclear factor-κB. Immunity 25, 701–715 (2006).
Vaughn, S. E., Kottyan, L. C., Munroe, M. E. & Harley, J. B. Genetic susceptibility to lupus: the biological basis of genetic risk found in B cell signaling pathways. J. Leuk. Biol. 92, 577–591 (2012).
Simon, A. R., Rai, U., Fanburg, B. L. & Cochran, B. H. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. Cell Physiol. 275, C1640–C1652 (1998).
Carballo, M. et al. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J. Biol. Chem. 274, 17580–17586 (1999).
Wegrzyn, J. et al. Function of mitochondrial Stat3 in cellular respiration. Science 323, 793–797 (2009).
Harada, T. et al. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity 40, 1–8 (2007).
Wu, T. et al. Shared signaling networks active in B cells isolated from genetically distinct mouse models of lupus. J. Clin. Invest. 117, 2186–2196 (2007).
de Beaucoudrey, L. et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205, 1543–1550 (2008).
Chiang, P. H. et al. mechanistic insights into impaired dendritic cell function by rapamycin: inhibition of Jak2/Stat4 signaling pathway. J. Immunol. 172, 1355–1363 (2004).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–304 (2011).
Desai, B. N., Myers, B. R. & Schreiber, S. L. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc. Natl Acad. Sci. USA. 99, 4319–4324 (2002).
Nambiar, M. P. et al. Oxidative stress is involved in the heat stress-induced downregulation of TCR ζ chain expression and TCR/CD3-mediated [Ca2+]i response in human T-lymphocytes. Cell. Immunol. 215, 151–161 (2002).
Fernandez, D., Bonilla, E., Mirza, N. & Perl, A. Rapamycin reduces disease activity and normalizes T-cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 54, 2983–2988 (2006).
Banki, K., Hutter, E., Gonchoroff, N. & Perl, A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J. Immunol. 162, 1466–1479 (1999).
Sunahori, K., Juang, Y. T. & Tsokos, G. C. Methylation status of CpG islands flanking a cAMP response element motif on the protein phosphatase 2Acα promoter determines CREB binding and activity. J. Immunol. 182, 1500–1508 (2009).
Juang, Y. T. et al. PP2A dephosphorylates Elf-1 and determines the expression of CD3ζ and FcRγ in human systemic lupus erythematosus T cells. J. Immunol. 181, 3658–3664 (2008).
Passam, F. H., Giannakopoulos, B., Mirarabshahi, P. & Krilis, S. A. Molecular pathophysiology of the antiphospholipid syndrome: The role of oxidative post-translational modification of β 2 glycoprotein I. J. Thromb. Haemost. 9, 275–282 (2011).
Otaki, N. et al. Identification of a lipid peroxidation product as the source of oxidation-specific epitopes recognized by anti-DNA autoantibodies. J. Biol. Chem. 285, 33834–33842 (2010).
Casciola-Rosen, L. A., Anhalt, G. & Rosen, A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 179, 1317–1330 (1994).
Nienhuis, H. L. A. et al. AGE and their receptor RAGE in systemic autoimmune diseases: An inflammation propagating factor contributing to accelerated atherosclerosis. Autoimmunity 42, 302–304 (2009).
Skaggs, B. J., Hahn, B. H. & McMahon, M. Accelerated atherosclerosis in patients with SLE—mechanisms and management. Nat. Rev. Rheumatol. 8, 214–223 (2012).
Khatoon, F., Moinuddin, Alam, K. & Ali, A. Physicochemical and immunological studies on 4-hydroxynonenal modified HSA: Implications of protein damage by lipid peroxidation products in the etiopathogenesis of SLE. Hum. Immunol. 73, 1132–1139 (2012).
Wang, G., Pierangeli, S. S., Papalardo, E., Ansari, G. A. S. & Khan, M. F. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: Correlation with disease activity. Arthritis Rheum. 62, 2064–2072 (2010).
Scofield, R. H. et al. Modification of lupus-associated 60-kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Rad. Biol. Med. 38, 719–728 (2005).
Ioannou, Y. et al. Naturally occurring free thiols within β2-glycoprotein I in vivo: nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress-induced cell injury. Blood 116, 1961–1970 (2010).
Balasubramanian, K. & Schroit, A. J. Characterization of phosphatidylserine-dependent β-2-glycoprotein I macrophage Interactions: implications for apoptotic cell clearance by phagocytes. J. Biol. Chem. 273, 29272–29277 (1998).
Sinicato, N. A., da Silva Cardoso, P. A. & Appenzeller, S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr. Cardiol. Rev. 9, 15–19 (2013).
Dwivedi, J. & Sarkar, P. D. Study of homocysteine, lipoprotein (a), lipid profile with oxidative stress in nephrotic syndrome and lupus nephritis. Res. J. Pharm. Biol. Chem. Sci. 1, 670–679 (2010).
Moroni, G. et al. Oxidative stress and homocysteine metabolism in patients with lupus nephritis. Lupus 19, 65–72 (2010).
Shao, X. et al. Inducible expression of kallikrein in renal tubular cells protects mice against spontaneous lupus nephritis. Arthritis Rheum. 65, 780–791 (2013).
Suwannaroj, S., Lagoo, A., Keisler, D. & McMurray, R. W. Antioxidants suppress mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus (SLE). Lupus 10, 258–265 (2001).
Bethunaickan, R. et al. Anti-tumor necrosis factor α treatment of interferon- α-induced murine lupus nephritis reduces the renal macrophage response but does not alter glomerular immune complex formation. Arthritis Rheum. 64, 3399–3408 (2012).
Mathis, K. W. et al. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension 59, 673–679 (2012).
Feng, X. et al. ApoE−/−Fas−/− C57BL/6 mice: a novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. J. Lipid Res. 48, 794–805 (2007).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am. J. Epidemiol. 145, 408–415 (1997).
Viigimaa, M. et al. Malondialdehyde-modified low-density lipoproteins as biomarker for atherosclerosis. Blood Press. 19, 164–168 (2010).
Ryan, M. J. The pathophysiology of hypertension in systemic lupus erythematosus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1258–R1267 (2009).
Yilmaz, S. et al. Association between serum total antioxidant status and coronary microvascular functions in patients with SLE. Echocardiography 29, 1218–1223 (2012).
Avalos, I. et al. Oxidative stress in systemic lupus erythematosus: Relationship to disease activity and symptoms. Lupus 16, 195–200 (2007).
Ioannou, Y. et al. Novel assays of thrombogenic pathogenicity in the antiphospholipid syndrome based on the detection of molecular oxidative modification of the major autoantigen β2-glycoprotein I. Arthritis Rheum. 63, 2774–2782 (2011).
Gilkeson, G. et al. Correlation of serum measures of nitric oxide production with lupus disease activity. J. Rheumatol. 26, 318–324 (1999).
Leitinger, N. The role of phospholipid oxidation products in inflammatory and autoimmune diseases: evidence from animal models and in humans. Subcell. Biochem. 49, 325–350 (2008).
Bergamo, P., Maurano, F. & Rossi, M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Rad. Biol. Med. 43, 71–79 (2007).
Kinscherf, R. et al. Cholesterol levels linked to abnormal plasma thiol concentrations and thiol/disulfide redox status in hyperlipidemic subjects. Free Rad. Biol. Med. 35, 1286–1292 (2003).
Costenbader, K. H., Kang, J. H. & Karlson, E. W. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am. J. Epidemiol. 172, 205–216 (2010).
Tam, L. S. et al. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: A double blind, placebo controlled pilot study. J. Rheumatol. 32, 275–282 (2005).
Puskas, F., Gergely, P., Banki, K. & Perl, A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 14, 1352–1361 (2000).
Cervera, R. et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 82, 299–308 (2003).
Montero, A. J. & Jassem, J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 71, 1385–1396 (2011).
Hiepe, F. et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 7, 170–178 (2011).
Engel, P., Gomez-Puerta, J. A., Ramos-Casals, M., Lozano, F. & Bosch, X. Therapeutic targeting of B cells for rheumatic autoimmune diseases. Pharmacol. Rev. 63, 127–156 (2011).
Pisetsky, D. S. & Vrabie, I. A. Antibodies to DNA: Infection or genetics? Lupus 18, 1176–1180 (2009).
Sorescu, D. et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation 105, 1429–1435 (2002).
Barry-Lane, P. A. et al. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J. Clin. Invest. 108, 1513–1522 (2001).
Banki, K., Hutter, E., Colombo, E., Gonchoroff, N. J. & Perl, A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J. Biol. Chem. 271, 32994–33001 (1996).
Acknowledgements
This work was supported in part by grants AI 048,079, AI 072,648, and AT004332 from the National Institutes of Health, the Alliance for Lupus Research, and the Central New York Community Foundation. The author is grateful to Mariana Kaplan (University of Michigan) and Mark Shlomchik (University of Pittsburgh) for helpful discussions and to Paul Phillips (State University of New York) for continued encouragement and support. Due to space limitations, important discoveries of oxidative stress research in SLE may have only been referenced through reviews.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary Table 1
Treatment approaches in SLE (DOC 44 kb)
Rights and permissions
About this article
Cite this article
Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol 9, 674–686 (2013). https://doi.org/10.1038/nrrheum.2013.147
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.147
This article is cited by
-
Systematic characterization of seed overlap microRNA cotargeting associated with lupus pathogenesis
BMC Biology (2022)
-
Mitochondrial reactive oxygen is critical for IL-12/IL-18-induced IFN-γ production by CD4+ T cells and is regulated by Fas/FasL signaling
Cell Death & Disease (2022)
-
Elucidation of the liver proteome in response to an antioxidant intake in rabbits
Egyptian Liver Journal (2021)
-
The impact of neutrophil extracellular trap from patients with systemic lupus erythematosus on the viability, CD11b expression and oxidative burst of healthy neutrophils
BMC Immunology (2021)
-
Type I interferons affect the metabolic fitness of CD8+ T cells from patients with systemic lupus erythematosus
Nature Communications (2021)