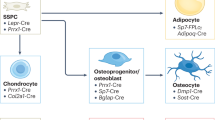
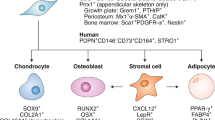
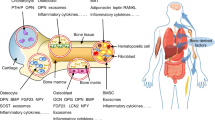
Abstract
Surprising new discoveries in the field of skeletal biology show that bone cells produce endocrine hormones that regulate phosphate and glucose homeostasis. In this Review, we examine the features of these new endocrine pathways and discuss their physiological importance in the context of our current understanding of energy metabolism and mineral homeostasis. Consideration of evolutionary and comparative biology provides clues that a key driving force for the emergence of these hormonal pathways was the development of a large, energy-expensive musculoskeletal system. Specialized bone cells also evolved and produced endocrine hormones to integrate the skeleton in global mineral and nutrient homeostasis. The recognition of bone as a true endocrine organ represents a fertile area for further research and should improve the diagnosis and treatment of metabolic diseases such as osteoporosis and diabetes mellitus.
Key Points
-
The endochondral skeleton evolved specialized bone cells (osteocytes), which produce endocrine hormones that integrate skeletal metabolism with global mineral and nutrient homeostasis
-
FGF23, made by osteocytes, regulates phosphate disposal from the body, providing an additional layer of control to aid parathyroid hormone in the maintenance of phosphate levels during bone resorption
-
Osteocalcin, produced by osteoblasts and osteocytes under the control of insulin, increases the efficiency of glucose utilization through its actions on the pancreas and adipocytes
-
Understanding the endocrine roles of the skeleton should improve the ability to diagnose and manage patients with a broad range of metabolic diseases, including osteoporosis and diabetes mellitus
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lu, Y. & Feng, J. Q. FGF23 in skeletal modeling and remodeling. Curr. Osteoporos Rep. 9, 103–108 (2011).
Clemens, T. L. & Karsenty, G. The osteoblast: an insulin target cell controlling glucose homeostasis. J. Bone Miner. Res. 26, 677–680 (2011).
Carroll, R. Paleontology: between fish and amphibian. Nature 373, 389–390 (1995).
Niedzwiedzki, G., Szrek, P., Narkiewicz, K., Narkiewicz, M. & Ahlberg, P. E. Tetrapod trackways from the early Middle Devonian period of Poland. Nature 463, 43–48 (2010).
Okabe, M. & Graham, A. The origin of the parathyroid gland. Proc. Natl Acad. Sci. USA 101, 17716–17719 (2004).
Botella, H., Blom, H., Dorka, M., Ahlberg, P. E. & Janvier, P. Jaws and teeth of the earliest bony fishes. Nature 448, 583–586 (2007).
Olsen, B. R., Reginato, A. M. & Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 16, 191–220 (2000).
Fernandez-Tresguerres-Hernandez-Gil, I., Alobera-Gracia, M. A., del- Canto-Pingarron, M. & Blanco-Jerez, L. Physiological bases of bone regeneration II. The remodeling process. Med. Oral Patol. Oral Cir. Bucal. 11, E151–E157 (2006).
Chen, J. H., Liu, C., You, L. & Simmons, C. A. Boning up on Wolff's Law: mechanical regulation of the cells that make and maintain bone. J. Biomech. 43, 108–118 (2010).
Bonewald, L. F. The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 (2011).
Pead, M. J., Suswillo, R., Skerry, T. M., Vedi, S. & Lanyon, L. E. Increased 3H-uridine levels in osteocytes following a single short period of dynamic bone loading in vivo. Calcif. Tissue Int. 43, 92–96 (1988).
Marotti, G., Cane, V., Palazzini, S. & Palumbo, C. Structure–function relationships in the osteocyte. Ital. J. Min. Electro. Metab. 4, 93–106 (1990).
Cardoso, L. et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J. Bone Miner. Res. 24, 597–605 (2009).
Herman, B. C., Cardoso, L., Majeska, R. J., Jepsen, K. J. & Schaffler, M. B. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone 47, 766–772 (2010).
Moester, M. J., Papapoulos, S. E., Lowik, C. W. & van Bezooijen, R. L. Sclerostin: current knowledge and future perspectives. Calcif. Tissue Int. 87, 99–107 (2010).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 (2011).
Denver, R. J. et al. Comparative endocrinology in the 21st century. Integr. Comp. Biol. 49, 339–348 (2009).
Scharrer, E. & Scharrer, B. On glandular nerve cells and neurosecretory organs in invertebrates and vertebrates. Biol. Rev. Camb. Philos. Soc. 12, 185–216 (1937).
Zera, A. J., Harshman, L. G. & Williams, T. D. Evolutionary endocrinology: The developing synthesis between endocrinology and evolutionary genetics. Ann. Rev. Ecol. Evol. System. 38, 793–817 (2007).
Brown, E. M. in The Parathyroids: Basic and Clinical Concepts (eds Bilezikian, J. P., Marcus, R. & Levine, M. A.) 167–182 (Academic Press, San Diego, California, USA, 2001).
Chan, S. J., Cao, Q. P. & Steiner, D. F. Evolution of the insulin superfamily: cloning of a hybrid insulin/insulin-like growth factor cDNA from amphioxus. Proc. Natl Acad. Sci. USA 87, 9319–9323 (1990).
Ebberink, R. H. M., Smit, A. B. & Vanminnen, J. The insulin family—evolution of structure and function in vertebrates and invertebrates. Biol. Bull. 177, 176–182 (1989).
Nagamatsu, S., Chan, S. J., Falkmer, S. & Steiner, D. F. Evolution of the insulin gene superfamily. Sequence of a preproinsulin-like growth factor cDNA from the Atlantic hagfish. J. Biol. Chem. 266, 2397–2402 (1991).
Bergwitz, C. & Juppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 61, 91–104 (2010).
Legros, R., Balmain, N. & Bonel, G. Age-related changes in mineral of rat and bovine cortical bone. Calcif. Tissue Int. 41, 137–144 (1987).
Beck, L. et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl Acad. Sci. USA 95, 5372–5377 (1998).
Farrow, E. G. & White, K. E. Recent advances in renal phosphate handling. Nat. Rev. Nephrol. 6, 207–217 (2010).
Burnett, C. H., Dent, C. E., Harper, C. & Warland, B. J. Vitamin D-resistant rickets. Analysis of twenty-four pedigrees with hereditary and sporadic cases. Am. J. Med. 36, 222–232 (1964).
Meyer, R. A. Jr, Meyer, M. H. & Gray, R. W. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J. Bone Miner. Res. 4, 493–500 (1989).
Morgan, J. M., Hawley, W. L., Chenoweth, A. I., Retan, W. J. & Diethelm, A. G. Renal transplantation in hypophosphatemia with vitamin D-resistant rickets. Arch. Intern. Med. 134, 549–552 (1974).
Nesbitt, T., Coffman, T. M., Griffiths, R. & Drezner, M. K. Crosstransplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic renal defect. J. Clin. Invest. 89, 1453–1459 (1992).
Francis, F. et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat. Genet. 11, 130–136 (1995).
White, K. E. et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 (2000).
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Quarles, L. D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 118, 3820–3828 (2008).
Feng, J. Q. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 (2006).
Lorenz-Depiereux, B. et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 38, 1248–1250 (2006).
Makitie, O. et al. Long-term clinical outcome and carrier phenotype in autosomal recessive hypophosphatemia caused by a novel DMP1 mutation. J. Bone Miner. Res. 25, 2165–2174 (2010).
Zhang, R. et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J. Bone Miner. Res. 26, 1047–1056 (2011).
Farrow, E. G. & White, K. E. Tumor-induced osteomalacia. Expert Rev. Endocrinol. Metab. 4, 435–442 (2009).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by Klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
Liu, S. et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17, 1305–1315 (2006).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Krajisnik, T. et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1α-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 195, 125–131 (2007).
Emmett, M. A comparison of clinically useful phosphorus binders for patients with chronic kidney failure. Kidney Int. Suppl. 90, S25–S32 (2004).
Mundy, G. R. & Guise, T. A. Hormonal control of calcium homeostasis. Clin. Chem. 45, 1347–1352 (1999).
Liu, S. et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–E49 (2006).
Ducy, P. et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000).
Takeda, S. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002).
Walther, D. J. et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76–76 (2003).
Yadav, V. K. et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138, 976–989 (2009).
Riggs, B. L., Khosla, S. & Melton, L. J. 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 23, 279–302 (2002).
Gorski, J. P. Biomineralization of bone: a fresh view of the roles of non-collagenous proteins. Front. Biosci. 16, 2598–2621 (2011).
Ducy, P. et al. Increased bone formation in osteocalcin-deficient mice. Nature 382, 448–452 (1996).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007).
Hauschka, P. V., Lian, J. B., Cole, D. E. & Gundberg, C. M. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev. 69, 990–1047 (1989).
Merle, B. & Delmas, P. D. Normal carboxylation of circulating osteocalcin (bone Gla-protein) in Paget's disease of bone. Bone Miner. 11, 237–245 (1990).
Ferron, M. et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 (2010).
Fulzele, K. et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142, 309–319 (2010).
Rached, M. T. et al. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J. Clin. Invest. 120, 357–368 (2010).
Kousteni, S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone 50, 437–443 (2012).
Delépine, M. et al. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott–Rallison syndrome. Nat. Genet. 25, 406–409 (2000).
Harding, H. P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Scheuner, D. et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001).
Oyadomari, S., Harding, H. P., Zhang, Y., Oyadomari, M. & Ron, D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell. Metab. 7, 520–532 (2008).
Yoshizawa, T. et al. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J. Clin. Invest. 119, 2807–2817 (2009).
Kode, A. et al. FoxO1 protein cooperates with ATF4 protein in osteoblasts to control glucose homeostasis. J. Biol. Chem. 287, 8757–8768 (2012).
Salo, J., Lehenkari, P., Mulari, M., Metsikko, K. & Vaananen, H. K. Removal of osteoclast bone resorption products by transcytosis. Science 276, 270–273 (1997).
Hinoi, E. et al. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J. Cell Biol. 183, 1235–1242 (2008).
Pasco, J. A. et al. β-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J. Bone Miner. Res. 19, 19–24 (2004).
Reid, I. R. et al. β-blocker use, BMD, and fractures in the study of osteoporotic fractures. J. Bone Miner. Res. 20, 613–618 (2005).
Schlienger, R. G., Kraenzlin, M. E., Jick, S. S. & Meier, C. R. Use of β-blockers and risk of fractures. JAMA 292, 1326–1332 (2004).
Turker, S., Karatosun, V. & Gunal, I. β-blockers increase bone mineral density. Clin. Orthop. Relat Res. 443, 73–74 (2006).
Egawa, K. et al. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J. Biol. Chem. 276, 10207–10211 (2001).
Kanazawa, I. et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 94, 45–49 (2009).
Kindblom, J. M. et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J. Bone Miner. Res. 24, 785–791 (2009).
Pittas, A. G., Harris, S. S., Eliades, M., Stark, P. & Dawson-Hughes, B. Association between serum osteocalcin and markers of metabolic phenotype. J. Clin. Endocrinol. Metab. 94, 827–832 (2009).
Saleem, U., Mosley, T. H. & Kullo, I. J. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 30, 1474–1478 (2010).
Acknowledgements
The authors wish to acknowledge grant support from the NIH: AR062074 (D. J. DiGirolamo); AR057868 (T. L. Clemens); and AR054447, AR055931 and AG032959 (S. Kousteni). T. L. Clemens is also the recipient of a Merit Review grant and Research Career Scientist Award from the Veterans Administration.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
DiGirolamo, D., Clemens, T. & Kousteni, S. The skeleton as an endocrine organ. Nat Rev Rheumatol 8, 674–683 (2012). https://doi.org/10.1038/nrrheum.2012.157
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.157
This article is cited by
-
The serine synthesis pathway drives osteoclast differentiation through epigenetic regulation of NFATc1 expression
Nature Metabolism (2024)
-
Targeting aging with the healthy skeletal system: The endocrine role of bone
Reviews in Endocrine and Metabolic Disorders (2023)
-
Circulating osteocalcin is associated with time in range and other metrics assessed by continuous glucose monitoring in type 2 diabetes
Diabetology & Metabolic Syndrome (2022)
-
Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus
Nature Reviews Endocrinology (2021)
-
Skeleton-vasculature chain reaction: a novel insight into the mystery of homeostasis
Bone Research (2021)