Abstract
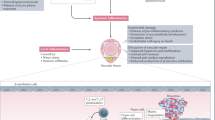
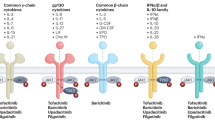
Cardiovascular disease represents a major source of extra-articular comorbidity in patients with rheumatoid arthritis (RA). A combination of traditional cardiovascular risk factors and RA-related factors accounts for the excess risk in RA. Among RA-related factors, chronic systemic inflammation has been implicated in the pathogenesis and progression of atherosclerosis. A growing body of evidence—mainly derived from observational databases and registries—suggests that specific RA therapies, including methotrexate and anti-TNF biologic agents, can reduce the risk of future cardiovascular events in patients with RA. The cardiovascular profile of other biologic therapies for the treatment of RA has not been adequately studied, including of investigational drugs that improve systemic inflammation but alter traditional cardiovascular risk factors. In the absence of large clinical trials adequately powered to detect differences in cardiovascular events between biologic drugs in RA, deriving firm conclusions on cardiovascular safety is challenging. Nevertheless, observational research using large registries has emerged as a promising approach to study the cardiovascular risk of emerging RA biologic therapies.
Key Points
-
Cardiovascular disease represents a major source of extra-articular comorbidity in patients with rheumatoid arthritis (RA)
-
Evidence derived from observational studies suggests that cardiovascular risk in people with RA might be reduced with the use of methotrexate or anti-TNF biologic agents, or both
-
In the absence of large randomized trials adequately powered to test cardiovascular outcomes, definitive conclusions about the cardiovascular risks and/or cardioprotective effects of biologic agents cannot yet be made
-
Despite inherent limitations of observational research, large registries of patients receiving long-term RA therapy represent a promising approach to studying the cardiovascular safety of emerging RA therapeutics
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Solomon, D. H. et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 107, 1303–1307 (2003).
Wolfe, F. & Michaud, K. The risk of myocardial infarction and pharmacologic and nonpharmacologic myocardial infarction predictors in rheumatoid arthritis: a cohort and nested case–control analysis. Arthritis Rheum. 58, 2612–2621 (2008).
Solomon, D. H. et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann. Rheum. Dis. 65, 1608–1612 (2006).
Meune, C., Touze, E., Trinquart, L. & Allanore, Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 48, 1309–1313 (2009).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Sattar, N., McCarey, D. W., Capell, H. & McInnes, I. B. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 108, 2957–2963 (2003).
Grundy, S. M., Pasternak, R., Greenland, P., Smith, S. Jr & Fuster, V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation 100, 1481–1492 (1999).
del Rincon, I. D., Williams, K., Stern, M. P., Freeman, G. L. & Escalante, A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 44, 2737–2745 (2001).
Solomon, D. H. et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann. Rheum. Dis. 69, 1920–1925 (2010).
Dessein, P. H. et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J. Rheumatol. 32, 435–442 (2005).
del Rincon, I., Freeman, G. L., Haas, R. W., O'Leary, D. H. & Escalante, A. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 52, 3413–3423 (2005).
Myasoedova, E. et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 70, 482–487 (2011).
Boyer, J. F., Gourraud, P. A., Cantagrel, A., Davignon, J. L. & Constantin, A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Joint Bone Spine 78, 179–183 (2011).
Book, C., Saxne, T. & Jacobsson, L. T. Prediction of mortality in rheumatoid arthritis based on disease activity markers. J. Rheumatol. 32, 430–434 (2005).
Maradit-Kremers, H., Nicola, P. J., Crowson, C. S., Ballman, K. V. & Gabriel, S. E. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 52, 722–732 (2005).
Dessein, P. H., Norton, G. R., Woodiwiss, A. J., Joffe, B. I. & Wolfe, F. Influence of nonclassical cardiovascular risk factors on the accuracy of predicting subclinical atherosclerosis in rheumatoid arthritis. J. Rheumatol. 34, 943–951 (2007).
Jacobsson, L. T. et al. Joint swelling as a predictor of death from cardiovascular disease in a population study of Pima Indians. Arthritis Rheum. 44, 1170–1176 (2001).
Wolfe, F., Michaud, K., Gefeller, O. & Choi, H. K. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 48, 1530–1542 (2003).
Farragher, T. M., Lunt, M., Bunn, D. K., Silman, A. J. & Symmons, D. P. Early functional disability predicts both all-cause and cardiovascular mortality in people with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann. Rheum. Dis. 66, 486–492 (2007).
Turesson, C., McClelland, R. L., Christianson, T. J. & Matteson, E. L. Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann. Rheum. Dis. 66, 70–75 (2007).
Del Rincon, I. et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 48, 1833–1840 (2003).
Wallberg-Jonsson, S., Johansson, H., Ohman, M. L. & Rantapaa-Dahlqvist, S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J. Rheumatol. 26, 2562–2571 (1999).
Goodson, N. J. et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 52, 2293–2299 (2005).
Rho, Y. H. et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 61, 1580–1585 (2009).
van Schaardenburg, D. et al. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J. Rheumatol. 20, 45–52 (1993).
Goodson, N. J. et al. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 46, 2010–2019 (2002).
Gonzalez, A. et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J. Rheumatol. 35, 1009–1014 (2008).
Liang, K. P. et al. Autoantibodies and the risk of cardiovascular events. J. Rheumatol. 36, 2462–2469 (2009).
Tomasson, G. et al. Effect of rheumatoid factor on mortality and coronary heart disease. Ann. Rheum. Dis. 69, 1649–1654 (2010).
Burut, D. F., Karim, Y. & Ferns, G. A. The role of immune complexes in atherogenesis. Angiology 61, 679–689 (2010).
del Val del Amo, N., Ibanez Bosch, R., Fito Manteca, C., Gutierrez Polo, R. & Loza Cortina, E. Anti-cyclic citrullinated peptide antibody in rheumatoid arthritis: relation with disease aggressiveness. Clin. Exp. Rheumatol. 24, 281–286 (2006).
Gerli, R. et al. Association of anti-cyclic citrullinated peptide antibodies with subclinical atherosclerosis in patients with rheumatoid arthritis. Ann. Rheum. Dis. 67, 724–725 (2008).
Lopez-Longo, F. J. et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 61, 419–424 (2009).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Valenzuela, A. et al. Association of HLA shared epitope with joint damage progression in rheumatoid arthritis. Hum. Immunol. Mar. 60, 250–254 (1999).
Gonzalez-Juanatey, C. et al. HLA-DRB1 status affects endothelial function in treated patients with rheumatoid arthritis. Am. J. Med. 114, 647–652 (2003).
Mattey, D. L. et al. Association of DRB1 shared epitope genotypes with early mortality in rheumatoid arthritis: results of eighteen years of followup from the early rheumatoid arthritis study. Arthritis Rheum. 56, 1408–1416 (2007).
Farragher, T. M. et al. Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum. 58, 359–369 (2008).
Toms, T. E. et al. Rheumatoid arthritis susceptibility genes associate with lipid levels in patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 1025–1032 (2011).
Libby, P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am. J. Med. 121 (Suppl. 1), S21–S31 (2008).
Pawlik, A. et al. The expansion of CD4+CD28− T cells in patients with rheumatoid arthritis. Arthritis Res. Ther. 5, R210–R213 (2003).
Gerli, R. et al. CD4+CD28- T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 109, 2744–2748 (2004).
Warrington, K. J. et al. Rheumatoid arthritis is an independent risk factor for multi-vessel coronary artery disease: a case control study. Arthritis Res. Ther. 7, R984–R991 (2005).
Liuzzo, G. et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101, 2883–2888 (2000).
Littler, A. J. et al. A distinct profile of six soluble adhesion molecules (ICAM-1, ICAM-3, VCAM-1, E-selectin, L-selectin and P-selectin) in rheumatoid arthritis. Br. J. Rheumatol. 36, 164–169 (1997).
Foster, W., Carruthers, D., Lip, G. Y. & Blann, A. D. Inflammatory cytokines, endothelial markers and adhesion molecules in rheumatoid arthritis: effect of intensive anti-inflammatory treatment. J. Thromb. Thrombolysis 29, 437–442 (2010).
Boutouyrie, P. et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39, 10–15 (2002).
Wong, M. et al. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 48, 81–89 (2003).
Roman, M. J. et al. Arterial stiffness in chronic inflammatory diseases. Hypertension 46, 194–199 (2005).
Kerekes, G. et al. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J. Rheumatol. 35, 398–406 (2008).
Pieringer, H., Schumacher, S., Stuby, U. & Biesenbach, G. Augmentation index and large-artery remodeling in patients with longstanding rheumatoid arthritis compared with healthy controls. Semin. Arthritis Rheum. 39, 163–169 (2009).
Saag, K. G. et al. American College of Rheumatology recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 59, 762–784 (2008).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 69, 964–975 (2010).
Chan, E. S. & Cronstein, B. N. Methotrexate—how does it really work? Nat. Rev. Rheumatol. 6, 175–178 (2010).
Choi, H. K., Hernan, M. A., Seeger, J. D., Robins, J. M. & Wolfe, F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 359, 1173–1177 (2002).
Goodson, N. The mortality association with DMARD use in early inflammatory arthritis [abstract]. Rheumatology (Oxford) 47 (Suppl.), ii49 (2008).
Landewe, R. B., van den Borne, B. E., Breedveld, F. C. & Dijkmans, B. A. Methotrexate effects in patients with rheumatoid arthritis with cardiovascular comorbidity. Lancet 355, 1616–1617 (2000).
Westlake, S. L. et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 49, 295–307 (2010).
van Halm, V. P., Nurmohamed, M. T., Twisk, J. W., Dijkmans, B. A. & Voskuyl, A. E. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res. Ther. 8, R151 (2006).
Naranjo, A. et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res. Ther. 10, R30 (2008).
Suissa, S., Bernatsky, S. & Hudson, M. Antirheumatic drug use and the risk of acute myocardial infarction. Arthritis Rheum. 55, 531–536 (2006).
Hochberg, M. C., Johnston, S. S. & John, A. K. The incidence and prevalence of extra-articular and systemic manifestations in a cohort of newly-diagnosed patients with rheumatoid arthritis between 1999 and 2006. Curr. Med. Res. Opin. 24, 469–480 (2008).
Greenberg, J. D. et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 576–582 (2011).
Reiss, A. B. et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 58, 3675–3683 (2008).
Popa, C., Netea, M. G., van Riel, P. L., van der Meer, J. W. & Stalenhoef, A. F. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 48, 751–762 (2007).
Barnabe, C., Martin, B. J. & Ghali, W. A. Systematic review and meta-analysis: Anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res. (Hoboken) 63, 522–529 (2011).
Westlake, S. L. et al. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 50, 518–531 (2011).
Dixon, W. G. et al. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor α therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 56, 2905–2912 (2007).
Levine, B., Kalman, J., Mayer, L., Fillit, H. M. & Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 323, 236–241 (1990).
Dutka, D. P., Elborn, J. S., Delamere, F., Shale, D. J. & Morris, G. K. Tumour necrosis factor α in severe congestive cardiac failure. Br. Heart J. 70, 141–143 (1993).
Chung, E. S., Packer, M., Lo, K. H., Fasanmade, A. A. & Willerson, J. T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107, 3133–3140 (2003).
Mann, D. L. et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109, 1594–1602 (2004).
Wolfe, F. & Michaud, K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am. J. Med. 116, 305–311 (2004).
Curtis, J. R. et al. Heart failure among younger rheumatoid arthritis and Crohn's patients exposed to TNF-α antagonists. Rheumatology (Oxford) 46, 1688–1693 (2007).
Cole, J., Busti, A. & Kazi, S. The incidence of new onset congestive heart failure and heart failure exacerbation in Veteran's Affairs patients receiving tumor necrosis factor α antagonists. Rheumatol. Int. 27, 369–373 (2007).
Setoguchi, S. et al. Tumor necrosis factor-α antagonist use and heart failure in elderly patients with rheumatoid arthritis. Am. Heart J. 156, 336–341 (2008).
Listing, J. et al. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 58, 667–677 (2008).
Klimiuk, P. A., Sierakowski, S., Domyslawska, I. & Chwiecko, J. Effect of etanercept on serum levels of soluble cell adhesion molecules (sICAM-1, sVCAM-1, and sE-selectin) and vascular endothelial growth factor in patients with rheumatoid arthritis. Scand. J. Rheumatol. 38, 439–444 (2009).
Hurlimann, D. et al. Anti-tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 106, 2184–2187 (2002).
Gonzalez-Juanatey, C. et al. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor α antibody. Arthritis Rheum. 51, 447–450 (2004).
Dixon, W. G. & Symmons, D. P. What effects might anti-TNFα treatment be expected to have on cardiovascular morbidity and mortality in rheumatoid arthritis? A review of the role of TNFα in cardiovascular pathophysiology. Ann. Rheum. Dis. 66, 1132–1136 (2007).
Solomon, D. H. et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 305, 2525–2531 (2011).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Emery, P. et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann. Rheum. Dis. 69, 510–516 (2010).
Gonzalez-Juanatey, C. et al. Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor α blocker therapy. Arthritis Rheum. 59, 1821–1824 (2008).
Kerekes, G. et al. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin. Rheumatol. 28, 705–710 (2009).
Mathieu, S. et al. Assessment of cardiovascular markers after 24 weeks of abatacept or rituximab therapy in patients with rheumatoid arthritis [abstract]. Arthritis Rheum. 62 (Suppl. 3), 388 (2010).
Raterman, H. et al. Rituximab alters the HDL particle from a pro-inflammatory into an anti-inflammatory property in good responding rheumatoid arthritis patients [abstract]. Ann. Rheum. Dis. 70 (Suppl. 3), 449 (2011).
Keystone, E. et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 56, 3896–3908 (2007).
Lewis, M. J. et al. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 120, 417–426 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
van Vollenhoven, R. et al. Long-term safety of rituximab: follow-up of the rheumatoid arthritis clinical trials and retreatment population [abstract]. Arthritis Rheum. 62 (Suppl.), S165 (2010).
Schiff, M. H. et al. The safety of anakinra in high-risk patients with active rheumatoid arthritis: six-month observations of patients with comorbid conditions. Arthritis Rheum. 50, 1529–1531 (2004).
Ikonomidis, I. et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 117, 2662–2669 (2008).
Maini, R. N. et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 54, 2817–2829 (2006).
Genovese, M. C. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 58, 2968–2980 (2008).
Nishimoto, N., Ito, K. & Takagi, N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod. Rheumatol. 20, 222–232 (2010).
Kawashiri, S. Y. et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol. Int. 31, 451–456 (2011).
Moriyama, M. et al. Tocilizumab increases serum lipids but does not promote atherosclerosis in patients with rheumatoid arthritis [abstract]. Ann. Rheum. Dis. 70 (Suppl. 3), 612 (2011).
Schultz, O. et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS ONE 5, e14328 (2010).
Ogata, A. et al. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann. Rheum. Dis. 70, 1164–1165 (2011).
Zampeli, E. et al. Treatment with tocilizumab improved arterial function in rheumatoid arthritis: a 6-month pilot study [abstract]. Ann. Rheum. Dis. 70 (Suppl. 3), 462 (2011).
Genovese, M. et al. Long-term safety of tocilizumab in rheumatoid arthritis clinical trials [abstract]. Ann. Rheum. Dis. 70, 609 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Fleischmann, R. et al. Safety and efficacy after 24 week dosing of the oral JAK inhibitor CP-690,550 as monotherapy in patients with active rheumatoid arthritis [abstract]. Arthritis Rheum. 60 (Suppl. 10), S718 (2009).
Kremer, J. M. et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 60, 1895–1905 (2009).
Tanaka, Y., Suzuki, M., Nakamura, H., Toyoizumi, S. & Zwillich, S. H. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and inadequate response to methotrexate. Arthritis Care Res. (Hoboken) 63, 1150–1158 (2011).
Weinblatt, M. E. et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 58, 3309–3318 (2008).
Bajpai, M., Chopra, P., Dastidar, S. G. & Ray, A. Spleen tyrosine kinase: a novel target for therapeutic intervention of rheumatoid arthritis. Expert Opin. Investig. Drugs. 17, 641–659 (2008).
Weinblatt, M. E. et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 363, 1303–1312 (2010).
Genovese, M. C. et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis Rheum. 63, 337–345 (2011).
Hilgendorf, I. et al. The oral spleen tyrosine kinase inhibitor fostamatinib attenuates inflammation and atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc Biol. 31, 1991–1999 (2011).
Dixon, W. G. et al. Serious infection following anti-tumor necrosis factor α therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies Arthritis Rheum. 56, 2896–2904 (2007).
Acknowledgements
Dr Greenberg received partial support for this work from the NIH (K23AR054412) and the Arthritis National Research Foundation. Dr Furer received partial support from the Arthritis Foundation.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to discussion of content and to review/editing of the manuscript before submission. J. D. Greenberg and V. Furer contributed equally to researching data for the article and to writing the article.
Corresponding author
Ethics declarations
Competing interests
J. D. Greenberg declares that he is a consultant for AstraZeneca, CORRONA, Novartis and Pfizer, and is a stock holder or director of CORRONA. M. Farkouh declares that he is a consultant for Genentech. V. Furer declares no competing interests.
Rights and permissions
About this article
Cite this article
Greenberg, J., Furer, V. & Farkouh, M. Cardiovascular safety of biologic therapies for the treatment of RA. Nat Rev Rheumatol 8, 13–21 (2012). https://doi.org/10.1038/nrrheum.2011.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.168
This article is cited by
-
Efficacy and safety of JAK inhibitors in rheumatoid arthritis: update for the practising clinician
Nature Reviews Rheumatology (2024)
-
Associations of vascular and bone status in arthritis patients
Scientific Reports (2021)
-
Common mechanisms and holistic care in atherosclerosis and osteoporosis
Arthritis Research & Therapy (2019)
-
Cardiovascular Disease Risk in Older Adults and Elderly Patients with Rheumatoid Arthritis: What Role Can Disease-Modifying Antirheumatic Drugs Play in Cardiovascular Risk Reduction?
Drugs & Aging (2019)
-
Comparison of the effects of exercise and anti-TNF treatment on cardiovascular health in rheumatoid arthritis: results from two controlled trials
Rheumatology International (2019)