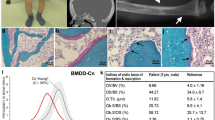
Abstract
Human disorders of hereditary and nonhereditary heterotopic ossification are conditions in which osteogenesis occurs outside of the skeleton, within soft tissues of the body. The resulting extraskeletal bone is normal. The aberration lies within the mechanisms that regulate cell-fate determination, directing the inappropriate formation of cartilage or bone, or both, in tissues such as skeletal muscle and adipose tissue. Specific gene mutations have been identified in two rare inherited disorders that are clinically characterized by extensive and progressive extraskeletal bone formation—fibrodysplasia ossificans progressiva and progressive osseous heteroplasia. In fibrodysplasia ossificans progressiva, activating mutations in activin receptor type-1, a bone morphogenetic protein type I receptor, induce heterotopic endochondral ossification, which results in the development of a functional bone organ system that includes skeletal-like bone and bone marrow. In progressive osseous heteroplasia, the heterotopic ossification leads to the formation of mainly intramembranous bone tissue in response to inactivating mutations in the GNAS gene. Patients with these diseases variably show malformation of normal skeletal elements, identifying the causative genes and their associated signaling pathways as key mediators of skeletal development in addition to regulating cell-fate decisions by adult stem cells.
Key Points
-
Heterotopic ossification is the formation of extraskeletal bone in soft connective tissues
-
The bone tissue that forms during heterotopic ossification is qualitatively normal
-
Two rare inherited forms of heterotopic ossification are fibrodysplasia ossificans progressiva (FOP) and progressive osseous heteroplasia (POH)
-
FOP is caused by a mutation in ACVR1, which encodes a bone morphogenetic protein type I receptor; POH is caused by a mutation in the GNAS locus
-
The genes and signaling pathways that are altered in these genetic disorders are key regulators of skeletal development and cell differentiation
-
Understanding the cellular mechanisms responsible for these rare disorders might lead to the development of therapeutic approaches relevant to common conditions of excessive and insufficient bone formation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others

References
Yang, Y. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed. Rosen, C. J.) 2–10 (American Society of Bone and Mineral Research, Washington, DC, 2008).
McCarthy, E. F. & Sundaram, M. Heterotopic ossification: a review. Skeletal Radiol. 34, 609–619 (2005).
Pignolo, R. J. & Foley, K. L. Nonhereditary heterotopic ossification. Implications for injury, arthropy, and aging. Clin. Rev. Bone Miner. Metab. 3, 261–266 (2005).
Forsberg, J. A. et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J. Bone Joint Surg. Am. 91, 1084–1091 (2009).
Neal, B., Gray, H., MacMahon, S. & Dunn, L. Incidence of heterotopic bone formation after major hip surgery. ANZ J. Surg. 72, 808–821 (2002).
Potter, B. K., Burns, T. C., Lacap, A. P., Granville, R. R. & Gajewski, D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J. Am. Acad. Orthop. Surg. 14 (10 Spec. No), S191–S197 (2006).
van Kuijk, A. A., Geurts, A. C. H. & van Kuppevelt, H. J. M. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord 40, 313–326 (2002).
Kaplan, F. S. & Shore, E. M. Progressive osseous heteroplasia. J. Bone Miner. Res. 15, 2084–2094 (2000).
Shore, E. M. & Kaplan, F. S. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP). Bone 43, 427–433 (2008).
MIM #135100. National Center for Biotechnology Information [online], (2010).
Kaplan, F. S. et al. The phenotype of fibrodysplasia ossificans progressiva. Clin. Rev. Bone Miner. Metab. 3, 183–188 (2005).
Kaplan, F. S. et al. Fibrodysplasia ossificans progressiva. Best Pract. Res. Clin. Rheumatol. 22, 191–205 (2008).
Kaplan, F. S. & Shore, E. M. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed. Rosen, C. J.) 442–446 (American Society for Bone and Mineral Research, Washington, DC, 2008).
Shore, E. M. & Kaplan, F. S. Fibrodysplasia ossificans progressiva and progressive osseous heteroplasia: two genetic disorders of heterotopic ossification. Clin. Rev. Bone Miner. Metab. 3, 257–259 (2005).
Shore, E. M. et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 38, 525–527 (2006).
Derynck, R. & Akhurst, R. J. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat. Cell Biol. 9, 1000–1004 (2007).
Eivers, E., Fuentealba, L. C. & De Robertis, E. M. Integrating positional information at the level of Smad1/5/8. Curr. Opin. Genet. Dev. 18, 304–310 (2008).
Heisenberg, C. P. & Solnica-Krezel, L. Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr. Opin. Genet. Dev. 18, 311–316 (2008).
Hogan, B. L. M. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10, 1580–1594 (1996).
Watabe, T. & Miyazono, K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 19, 103–115 (2009).
Wu, M. Y. & Hill, C. S. TGF-β superfamily signaling in embryonic development and homeostasis. Dev. Cell 16, 329–343 (2009).
Xu, J., Lamouille, S. & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 (2009).
Urist, M. R. Bone: formation by autoinduction. Science 150, 893–899 (1965).
Derynck, R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Huse, M. et al. The TGFβ receptor activation process: an inhibitor- to substrate-binding switch. Mol. Cell 8, 671–682 (2001).
Krause, C., de Gorter, D. J. J., Karperien, M. & ten Dijke, P. in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed. Rosen, C. J.) 10–16 (American Society of Bone and Mineral Research Washington, DC, (2008).
Schmierer, B. & Hill, C. S. TGF-β–SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 8, 970–982 (2007).
Shi, Y. & Massague, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
ten Dijke, P. & Hill, C. S. New insights into TGF-β–Smad signalling. Trends Biochem. Sci. 29, 265–273 (2004).
Guo, X. & Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 19, 71–88 (2009).
Moustakas, A. & Heldin, C. H. Non-Smad TGF-β signals. J. Cell Sci. 118, 3573–3584 (2005).
Nohe, A. et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 277, 5330–5338 (2002).
Chen, Y. G. et al. Determinants of specificity in TGF-β signal transduction. Genes Dev. 12, 2144–2152 (1998).
Little, S. C. & Mullins, M. C. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes that pattern the dorsoventral axis. Nat. Cell Biol. 11, 637–643 (2009).
Massague, J. How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 1, 169–178 (2000).
Gazzerro, E. & Canalis, E. Bone morphogenetic proteins and their antagonists. Rev. Endocr. Metab. Disord. 7, 51–65 (2006).
Yu, P. B. et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat. Med. 14, 1363–1369 (2008).
Zhang, D. et al. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J. Bone Miner. Res. 18, 1593–1604 (2003).
Billings, P. C. et al. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J. Bone Miner. Res. 23, 305–313 (2008).
Fiori, J. L., Billings, P. C., Serrano de la Pena, L. S., Kaplan, F. S. & Shore, E. M. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP). J. Bone Miner. Res. 21, 902–909 (2006).
Serrano de la Pena, L. S. et al. Fibrodysplasia ossificans progressiva (FOP), a disorder of ectopic osteogenesis, misregulates cell surface expression and trafficking of BMPRIA. J. Bone Miner. Res. 20, 1168–1176 (2005).
Shafritz, A. B. et al. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N. Engl. J. Med. 335, 555–561 (1996).
Fukuda, T. et al. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J. Biol. Chem. 284, 7149–7156 (2009).
Shen, Q. et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J. Clin. Invest. 119, 3462–3472 (2009).
Gu, Z. et al. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126, 2551–2561 (1999).
Mishina, Y., Crombie, R., Bradley, A. & Behringer, R. R. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev. Biol. 213, 314–326 (1999).
Pignolo, R. J., Suda, R. K. & Kaplan, F. S. The fibrodysplasia ossificans progressiva lesion. Clin. Rev. Bone Miner. Metab. 3, 195–200 (2005).
Cohen, R. B. et al. The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. A study of forty-four patients. J. Bone Joint Surg. Am. 75, 215–219 (1993).
Rocke, D. M., Zasloff, M., Peeper, J., Cohen, R. B. & Kaplan, F. S. Age- and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clin. Orthop. Relat. Res. 301, 243–248 (1994).
Lanchoney, T. F., Cohen, R. B., Rocke, D. M., Zasloff, M. A. & Kaplan, F. S. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J. Pediatr. 126, 762–764 (1995).
Scarlett, R. F. et al. Influenza-like viral illnesses and flare-ups of fibrodysplasia ossificans progressiva. Clin. Orthop. Rel. Res. 423, 275–279 (2004).
Gannon, F. H., Valentine, B. A., Shore, E. M., Zasloff, M. A. & Kaplan, F. S. Acute lymphocytic infiltration in an extremely early lesion of fibrodysplasia ossificans progressiva. Clin. Orthop. Rel. Res. 346, 19–25 (1998).
Glaser, D. L. et al. In vivo somatic cell gene transfer of an engineered noggin mutein prevents BMP4-induced heterotopic ossification. J. Bone Joint Surg. Am. 85A, 2332–2342 (2003).
Hegyi, L. et al. Stromal cells of fibrodysplasia ossificans progressiva lesions express smooth muscle lineage markers and the osteogenic transcription factor Runx2/Cbfa-1: clues to a vascular origin of heterotopic ossification? J. Pathol. 201, 141–148 (2003).
Kaplan, F. S. et al. Immunological features of fibrodysplasia ossificans progessiva and the dysregulated BMP4 pathway. Clin. Rev. Bone Miner. Metab. 3, 189–193 (2005).
Kaplan, F. S. et al. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J. Bone Joint Surg. Am. 75, 220–230 (1993).
Lounev, V. Y. et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 91, 652–663 (2009).
Kaplan, F. S. et al. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J. Bone Joint Surg. Am. 89A, 347–357 (2007).
Kaplan, F. S., Strear, C. M. & Zasloff, M. A. Radiographic and scintigraphic features of modeling and remodeling in the heterotopic skeleton of patients who have fibrodysplasia ossificans progressiva. Clin. Orthop. Rel. Res. 304, 238–247 (1994).
Kaplan, F. et al. Urinary basic fibroblast growth factor. A biochemical marker for preosseous fibroproliferative lesions in patients with fibrodysplasia ossificans progressiva. Clin. Orthop. Rel. Res. 346, 59–65 (1998).
Lutwak, L. Myositis ossificans progressiva. Mineral, metabolic and radioactive calcium studies of the effects of hormones. Am. J. Med. 37, 269–293 (1964).
Ferguson, C., Alpern, E., Miclau, T. & Helms, J. A. Does adult fracture repair recapitulate embryonic skeletal formation? Mech. Dev. 87, 57–66 (1999).
Vortkamp, A. et al. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech. Dev. 71, 65–76 (1998).
Dean, D. B., Watson, J. T., Moed, B. R. & Zhang, Z. Role of bone morphogenetic proteins and their antagonists in healing of bone fracture. Front. Biosci. 14, 2878–2888 (2009).
Gerstenfeld, L. C., Cullinane, D. M., Barnes, G. L., Graves, D. T. & Einhorn, T. A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 88, 873–884 (2003).
Kloen, P. et al. BMP signaling components are expressed in human fracture callus. Bone 33, 362–371 (2003).
Tsuji, K. et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 38, 1424–1429 (2006).
Einhorn, T. A. & Kaplan, F. S. Traumatic fractures of heterotopic bone in patients who have fibrodysplasia ossificans progressiva. A report of 2 cases. Clin. Orthop. Rel. Res. 308, 173–177 (1994).
Connor, J. M. & Evans, D. A. Fibrodysplasia ossificans progressiva. The clinical features and natural history of 34 patients. J. Bone Joint Surg. Br. 64, 76–83 (1982).
Deirmengian, G. K. et al. Proximal tibial osteochondromas in patients with fibrodysplasia ossificans progressiva. J. Bone Joint Surg. Am. 90, 366–374 (2008).
Schaffer, A. A. et al. Developmental anomalies of the cervical spine in patients with fibrodysplasia ossificans progressiva are distinctly different from those in patients with Klippel-Feil syndrome —Clues from the BMP signaling pathway. Spine 30, 1379–1385 (2005).
Seemann, P., Mundlos, S. & Lehmann, K. In Bone Morphogenetic Proteins: From Local to System Therapeutics (eds. Vukicevic, S. & Sampath, K. T.) 141–159 (Birkhäuser, Basel, 2008).
Kaplan, F. S. et al. Classic and atypical FOP phenotypes are caused by mutations in the BMP type I receptor ACVR1. Hum. Mutat. 30, 379–390 (2009).
Wozney, J. M. et al. Novel regulators of bone formation: molecular clones and activities. Science 242, 1528–1534 (1988).
Deng, Z. L. et al. Regulation of osteogenic differentiation during skeletal development. Front. Biosci. 13, 2001–2021 (2008).
Furuta, Y. & Hogan, B. L. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 12, 3764–3775 (1998).
Lawson, K. A. et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424–436 (1999).
Mitu, G. M., Wang, S. & Hirschberg, R. BMP7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am. J. Physiol. Renal Physiol. 293, F1641–F1648 (2007).
Morty, R. E. et al. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 27, 1072–1078 (2007).
Valdimarsdottir, G. et al. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation 106, 2263–2270 (2002).
de Sousa Lopes, S. M. et al. BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev. 18, 1838–1849 (2004).
Dudas, M., Sridurongrit, S., Nagy, A., Okazaki, K. & Kaartinen, V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech. Dev. 121, 173–182 (2004).
Kaartinen, V. et al. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development 131, 3481–3490 (2004).
Kevenaar, M. E. et al. Variants in the ACVR1 gene are associated with AMH levels in women with polycystic ovary syndrome. Hum. Reprod. 24, 241–249 (2009).
Komatsu, Y., Scott, G., Nagy, A., Kaartinen, V. & Mishina, Y. BMP type I receptor ALK2 is essential for proper patterning at late gastrulation during mouse embryogenesis. Dev. Dyn. 236, 512–517 (2007).
Rajagopal, R. et al. Functions of the type 1 BMP receptor Acvr1 (Alk2) in lens development: cell proliferation, terminal differentiation, and survival. Invest. Ophthal. Vis. Sci. 49, 4953–4960 (2008).
MIM #166350, National Center for Biotechnological Information. [online], (2010).
Kaplan, F. S. et al. Progressive osseous heteroplasia: a distinct developmental disorder of heterotopic ossification. Two new case reports and follow-up of three previously reported cases. J. Bone Joint Surg. Am. 76, 425–436 (1994).
Shore, E. M., Feldman, G. J., Xu, M. & Kaplan, F. S. The genetics of fibrodysplasia ossificans progressiva. Clin. Rev. Bone Miner. Metab. 3, 201–204 (2005).
Shore, E. M. et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N. Engl. J. Med. 346, 99–106 (2002).
Adegbite, N. S., Xu, M., Kaplan, F. S., Shore, E. M. & Pignolo, R. J. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am. J. Med. Genet. A 146A, 1788–1796 (2008).
Plagge, A., Kelsey, G. & Germain-Lee, E. L. Physiological functions of the imprinted Gnas locus and its protein variants Gαs and XLαs in human and mouse. J. Endocrinol. 196, 193–214 (2008).
Rubin, M. R. & Levine, M. A. in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed. Rosen, C. J.) 354–361 (American Society for Bone and Mineral Research, Washington, DC, 2008).
Weinstein, L. S., Chen, M., Xie, T. & Liu, J. Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol. Sci. 27, 260–266 (2006).
Weinstein, L. S., Xie, T., Zhang, Q. H. & Chen, M. Studies of the regulation and function of the Gsα gene Gnas using gene targeting technology. Pharmacol. Ther. 115, 271–291 (2007).
Bastepe, M. & Jüppner, H. GNAS locus and pseudohypoparathyroidism. Horm. Res. 63, 65–74 (2005).
Yeh, G. L. et al. GNAS1 mutation and Cbfa1 misexpression in a child with severe congenital platelike osteoma cutis. J. Bone Miner. Res. 15, 2063–2073 (2000).
Eddy, M. C. et al. Deficiency of the α-subunit of the stimulatory G protein and severe extraskeletal ossification. J. Bone Miner. Res. 15, 2074–2083 (2000).
Huso, D. L., McGuire, S. & Germaine-Lee, E. L. Heterotopic subcutaneous ossifications in a mouse model of Albright hereditary osteodystophy. Presented at The Endocrine Society 89th Annual Meeting, Toronto, Canada, (2007).
Plagge, A. et al. The imprinted signaling protein XLαs is required for postnatal adaptation to feeding. Nat. Genet. 36, 818–826 (2004).
Bastepe, M. et al. A form of Jansen's metaphyseal chondrodysplasia with limited metabolic and skeletal abnormalities is caused by a novel activating parathyroid hormone (PTH)/PTH-related peptide receptor mutation. J. Clin. Endocrinol. Metab. 89, 3595–3600 (2004).
Sakamoto, A., Chen, M., Kobayashi, T., Kronenberg, H. M. & Weinstein, L. S. Chondrocyte-specific knockout of the G protein Gsα leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J. Bone Miner. Res. 20, 663–671 (2005).
Sakamoto, A. et al. Deficiency of the G-protein α-subunit Gsα in osteoblasts leads to differential effects on trabecular and cortical bone. J. Biol. Chem. 289, 21369–21375 (2005).
Wu, J. Y., Scadden, D. T. & Kronenberg, H. M. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J. Bone Miner. Res. 24, 759–764 (2009).
Kaplan, F. S., Groppe, J. C. & Shore, E. M. When one skeleton is enough: approaches and strategies for the treatment of fibrodysplasia ossificans progressiva (FOP). Drug Discov. Today Ther. Strateg. 5, 255–262 (2008).
Aydin, C. et al. Extralarge XLαs (XXLαs), a variant of stimulatory G protein α-subunit (Gsα), is a distinct, membrane-anchored GNAS product that can mimic Gsα. Endocrinology 150, 3567–3575 (2009).
Bastepe, M. et al. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc. Natl Acad. Sci. USA 101, 14794–14799 (2004).
Michienzi, S. et al. GNAS transcripts in skeletal progenitors: evidence for random asymmetric allelic expression of Gsα. Hum. Mol. Genet. 16, 1921–1930 (2007).
Yang, D. C. et al. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS ONE 3, e1540 (2008).
Zhang, S., Xu, M., Kaplan, F. S., Pignolo, F. S. & Shore, E. M. G protein–cAMP pathway regulates early stage embryonic stem cell-derived osteoblast differentiation. J. Bone Miner. Res. 24, S115 (2009).
Acknowledgements
We thank the members of our research laboratories and our many collaborators and colleagues for their contributions, notably J. Haupt and R. Mauck who generously provided figures (Figure 2 and Figure 4, respectively) that were adapted for this manuscript. We also thank the NIH/NIAMS-supported Penn Center for Musculoskeletal Disorders (AR050950). This work was supported in part by the Center for Research in FOP and Related Disorders, the International FOP Association (IFOPA), the Ian Cali Endowment, the Weldon Family Endowment, the Isaac and Rose Nassau Professorship of Orthopedic Molecular Medicine, the Rita Allen Foundation, and by grants from the National Institutes of Health (R01- AR41916 and R01-AR046831).
Charles P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
E. M. Shore researched the data for the article and wrote the article. Both E. M. Shore and F. S. Kaplan contributed equally to discussion of content and to reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Shore, E., Kaplan, F. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol 6, 518–527 (2010). https://doi.org/10.1038/nrrheum.2010.122
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.122
This article is cited by
-
TNFα-dependent mTOR activity is required for tenotomy-induced ectopic ossification in mice
Journal of Bone and Mineral Metabolism (2023)
-
Fibrodysplasia ossificans progressiva – wenn sich plötzlich Knochen im Muskel bilden
Monatsschrift Kinderheilkunde (2023)
-
Empfehlungen zur Versorgung von Patient:innen mit FOP
Die Orthopädie (2023)
-
The Pharmacokinetic Profile of Palovarotene: An Open-Label Phase I Trial Investigating the Effect of Food and Potential for Drug–Drug Interaction in Healthy Participants
European Journal of Drug Metabolism and Pharmacokinetics (2023)
-
Enhancer hijacking at the ARHGAP36 locus is associated with connective tissue to bone transformation
Nature Communications (2023)