Abstract
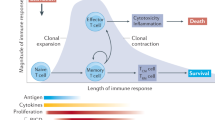
Invariant natural killer T (iNKT) cells are an innate T-cell lineage known to recognize a range of endogenously derived and exogenously derived glycolipid antigens. Advances in our understanding of this T-cell subset have enabled researchers to investigate the immunomodulatory activity of iNKT cell ligands in experimental models of diseases such as cancer, allergy and chronic inflammatory joint disease. To a large extent, the ability of iNKT cells to regulate such disease models has been ascribed to their capacity to promote a polarized cytokine environment, which is understood to skew adaptive immune responses. In this Review, we discuss the current understanding of how iNKT-cell polarization is regulated and relate this basic theory to the proposed role for iNKT cells in models of rheumatologic disease.
Key Points
-
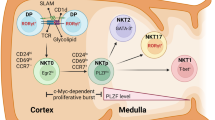
Invariant natural killer T (iNKT) cells are positively selected in the thymus by CD1d-expressing T-cell precursors
-
iNKT cells in mice and humans constitute a heterogeneous population of cells capable of producing a broad spectrum of cytokines
-
Cytokine production by iNKT cells is regulated by both antigen-dependent and antigen-independent mechanisms; these modes of activation are not necessarily mutually exclusive
-
iNKT cell activation in rheumatic disease might be dependent on the presence of glycolipid antigens of host origin as well as exogenously derived antigens
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bix, M. & Locksley, R. M. Natural T cells. Cells that co-express NKRP-1 and TCR. J. Immunol. 155, 1020–1022 (1995).
Bendelac, A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995).
MacDonald, H. R. NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J. Exp. Med. 182, 633–638 (1995).
Imai, K. et al. Sequence and expression of transcripts of the T-cell antigen receptor alpha-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma. Proc. Natl Acad. Sci. USA 83, 8708–8712 (1986).
Godfrey, D. I., MacDonald, H. R., Kronenberg, M., Smyth, M. J. & Van Kaer, L. NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 (2004).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 (2005).
Rolf, J. et al. Molecular profiling reveals distinct functional attributes of CD1d-restricted natural killer (NK) T cell subsets. Mol. Immunol. 45, 2607–2620 (2008).
Taniguchi, M., Harada, M., Kojo, S., Nakayama, T. & Wakao, H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21, 483–513 (2003).
Terabe, M. & Berzofsky, J. A. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 28, 491–496 (2007).
Lisbonne, M. et al. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171, 1637–1641 (2003).
Akbari, O. et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 9, 582–588 (2003).
Seino, K. I. et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc. Natl Acad. Sci. USA 98, 2577–2581 (2001).
Ikehara, Y. et al. CD4+ Vα14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J. Clin. Invest. 105, 1761–1767 (2000).
Campos, R. A. et al. Cutaneous immunization rapidly activates liver invariant Vα14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 198, 1785–1796 (2003).
Askenase, P. W. et al. TLR-dependent IL-4 production by invariant Vα14+Jα18+ NKT cells to initiate contact sensitivity in vivo. J. Immunol. 175, 6390–6401 (2005).
Campos, R. A. et al. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J. Immunol. 177, 3686–3694 (2006).
Sharif, S. et al. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat. Med. 7, 1057–1062 (2001).
Singh, A. K. et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 194, 1801–1811 (2001).
Chiba, A. et al. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of α-galactosylceramide. Arthritis Rheum. 50, 305–313 (2004).
Yang, J. Q. et al. Repeated alpha-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. J. Immunol. 171, 4439–4446 (2003).
Miyamoto, K., Miyake, S. & Yamamura, T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534 (2001).
Ueno, Y. et al. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of Valpha14 natural killer T cells in mice. Inflamm. Bowel Dis. 11, 35–41 (2005).
Forestier, C. et al. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J. Immunol. 178, 1415–1425 (2007).
Grajewski, R. S. et al. Activation of invariant NKT cells ameliorates experimental ocular autoimmunity by a mechanism involving innate IFN-γ production and dampening of the adaptive Th1 and Th17 responses. J. Immunol. 181, 4791–4797 (2008).
Yamamura, T. et al. NKT cell-stimulating synthetic glycolipids as potential therapeutics for autoimmune disease. Curr. Top. Med. Chem. 4, 561–567 (2004).
Benlagha, K., Wei, D. G., Veiga, J., Teyton, L. & Bendelac, A. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202, 485–492 (2005).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Sandberg, J. K., Stoddart, C. A., Brilot, F., Jordan, K. A. & Nixon, D. F. Development of innate CD4+ α-chain variable gene segment 24 (Vα24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc. Natl Acad. Sci. USA 101, 7058–7063 (2004).
Hager, E., Hawwari, A., Matsuda, J. L., Krangel, M. S. & Gapin, L. Multiple constraints at the level of TCRα rearrangement impact Vα14i NKT cell development. J. Immunol. 179, 2228–2234 (2007).
Egawa, T. et al. Genetic evidence supporting selection of the Vα14i NKT cell lineage from double-positive thymocyte precursors. Immunity 22, 705–716 (2005).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Speak, A. O. et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc. Natl Acad. Sci. USA 104, 5971–5976 (2007).
Porubsky, S. et al. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl Acad. Sci. USA 104, 5977–5982 (2007).
Godfrey, D. I. & Berzins, S. P. Control points in NKT-cell development. Nat. Rev. Immunol. 7, 505–518 (2007).
Pellicci, D. G. et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1−CD4+ CD1d-dependent precursor stage. J. Exp. Med. 195, 835–844 (2002).
Stetson, D. B. et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 (2003).
Tanaka, S. et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity 24, 689–701 (2006).
Scheu, S. et al. Activation of the integrated stress response during T helper cell differentiation. Nat. Immunol. 7, 644–651 (2006).
Townsend, M. J. et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20, 477–494 (2004).
Kim, H. Y., Kim, S. & Chung, D. H. FcγRIII engagement provides activating signals to NKT cells in antibody-induced joint inflammation. J. Clin. Invest. 116, 2484–2492 (2006).
Wang, Z. Y. et al. Regulation of Th2 cytokine expression in NKT cells: unconventional use of STAT6, GATA-3, and NFAT2. J. Immunol. 176, 880–888 (2006).
Crowe, N. Y. et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202, 1279–1288 (2005).
Michel, M. L. et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc. Natl Acad. Sci. USA 105, 19845–19850 (2008).
Pichavant, M. et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J. Exp. Med. 205, 385–393 (2008).
Michel, M. L. et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001 (2007).
Doisne, J. M. et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor γt+ and respond preferentially under inflammatory conditions. J. Immunol. 183, 2142–2149 (2009).
Gumperz, J. E., Miyake, S., Yamamura, T. & Brenner, M. B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195, 625–636 (2002).
Lee, P. T., Benlagha, K., Teyton, L. & Bendelac, A. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 195, 637–641 (2002).
Coquet, J. M. et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4−NK1.1− NKT cell population. Proc. Natl Acad. Sci. USA 105, 11287–11292 (2008).
Schmieg, J., Yang, G., Franck, R. W. & Tsuji, M. Superior protection against malaria and melanoma metastases by a C-glycoside analog of the natural killer T cell ligand α-Galactosylceramide. J. Exp. Med. 198, 1631–1641 (2003).
Oki, S., Tomi, C., Yamamura, T. & Miyake, S. Preferential Th2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int. Immunol. 17, 1619–1629 (2005).
Matsuda, J. L. et al. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc. Natl Acad. Sci. USA 100, 8395–8400 (2003).
Yu, K. O. et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc. Natl Acad. Sci. USA 102, 3383–3388 (2005).
Im, J. S. et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity 30, 888–898 (2009).
Bai, L. et al. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen αGalCer. Proc. Natl Acad. Sci. USA 106, 10254–10259 (2009).
Turley, S. J. et al. Transport of peptide–MHC class II complexes in developing dendritic cells. Science 288, 522–527 (2000).
Buatois, V. et al. MHC class II–peptide complexes in dendritic cell lipid microdomains initiate the CD4 Th1 phenotype. J. Immunol. 171, 5812–5819 (2003).
Amprey, J. L. et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200, 895–904 (2004).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Kinjo, Y. et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7, 978–986 (2006).
Brigl, M., Bry, L., Kent, S. C., Gumperz, J. E. & Brenner, M. B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 (2003).
Paget, C. et al. Activation of invariant NKT cells by Toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 (2007).
Salio, M. et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl Acad. Sci. USA 104, 20490–20495 (2007).
Nagarajan, N. A. & Kronenberg, M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 178, 2706–2713 (2007).
Tyznik, A. J. et al. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 181, 4452–4456 (2008).
Wesley, J. D., Tessmer, M. S., Chaukos, D. & Brossay, L. NK cell-like behavior of Vα14i NK T cells during MCMV infection. PLoS Pathog. 4, e1000106 (2008).
van den Elzen, P. et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437, 906–910 (2005).
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002).
Kim, E. Y. et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 14, 633–640 (2008).
Terashima, A. et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 205, 2727–2733 (2008).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Barrat, F. J., Meeker, T., Chan, J. H., Guiducci, C. & Coffman, R. L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 37, 3582–3586 (2007).
Barrat, F. J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005).
Berland, R. et al. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 25, 429–440 (2006).
Savarese, E. et al. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood 107, 3229–3234 (2006).
Lau, C. M. et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 (2005).
Abdollahi-Roodsaz, S. et al. Shift from Toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 58, 3753–3764 (2008).
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Abdollahi-Roodsaz, S. et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–2967 (2007).
Huang, Q., Ma, Y., Adebayo, A. & Pope, R. M. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 56, 2192–2201 (2007).
Huang, Q. Q. et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J. Immunol. 182, 4965–4973 (2009).
Ospelt, C. et al. Overexpression of Toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 58, 3684–3692 (2008).
Roelofs, M. F. et al. The expression of Toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and co-stimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 52, 2313–2322 (2005).
Godfrey, D. I. & Kronenberg, M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
Coppieters, K. et al. A single early activation of invariant NK T cells confers long-term protection against collagen-induced arthritis in a ligand-specific manner. J. Immunol. 179, 2300–2309 (2007).
Miellot, A. et al. Activation of invariant NK T cells protects against experimental rheumatoid arthritis by an IL-10-dependent pathway. Eur. J. Immunol. 35, 33704–33713 (2005).
Yang, J. Q. et al. CD1d deficiency exacerbates inflammatory dermatitis in MRL-lpr/lpr mice. Eur. J. Immunol. 34, 1723–1732 (2004).
Chiba, A., Kaieda, S., Oki, S., Yamamura, T. & Miyake, S. The involvement of Vα14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum. 52, 1941–1948 (2005).
Yang, J. Q. et al. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J. Immunol. 171, 2142–2153 (2003).
Chan, O. T. et al. Deficiency in β2-microglobulin, but not CD1, accelerates spontaneous lupus skin disease while inhibiting nephritis in MRL-Faslpr mice: an example of disease regulation at the organ level. J. Immunol. 167, 2985–2990 (2001).
Zeng, D., Liu, Y., Sidobre, S., Kronenberg, M. & Strober, S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J. Clin. Invest. 112, 1211–1222 (2003).
Forestier, C. et al. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White) F1 mice. J. Immunol. 175, 763–770 (2005).
Postól, E. et al. Long-term administration of IgG2a anti-NK1.1 monoclonal antibody ameliorates lupus-like disease in NZB/W mice in spite of an early worsening induced by an IgG2a-dependent BAFF/BLyS production. Immunology 125, 184–196 (2008).
Singh, A. K. et al. The natural killer T cell ligand α-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur. J. Immunol. 35, 1143–1154 (2005).
Green, M. R. et al. Natural killer T cells in families of patients with systemic lupus erythematosus: their possible role in regulation of IGG production. Arthritis Rheum. 56, 303–310 (2007).
Dai, Z. et al. Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus. J. Exp. Med. 206, 793–805 (2009).
Wither, J. et al. Reduced proportions of natural killer T cells are present in the relatives of lupus patients and are associated with autoimmunity. Arthritis Res. Ther. 10, R108 (2008).
Esteban, L. M. et al. Genetic control of NKT cell numbers maps to major diabetes and lupus loci. J. Immunol. 171, 2873–2878 (2003).
Fletcher, J. M. et al. Congenic analysis of the NKT cell control gene Nkt2 implicates the peroxisomal protein Pxmp4. J. Immunol. 181, 3400–3412 (2008).
Jordan, M. A., Fletcher, J. M., Pellicci, D. & Baxter, A. G. Slamf1, the NKT cell control gene Nkt1. J. Immunol. 178, 1618–1627 (2007).
Loh, C. et al. Dissociation of the genetic loci leading to B1a and NKT cell expansions from autoantibody production and renal disease in B6 mice with an introgressed New Zealand Black chromosome 4 interval. J. Immunol. 178, 1608–1617 (2007).
Tsukamoto, K. et al. Aberrant genetic control of invariant TCR-bearing NKT cell function in New Zealand mouse strains: possible involvement in systemic lupus erythematosus pathogenesis. J. Immunol. 180, 4530–4539 (2008).
Singh, N. et al. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163, 2373–2377 (1999).
Burdin, N., Brossay, L. & Kronenberg, M. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29, 2014–2025 (1999).
Kim, H. Y. et al. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor β1 production. J. Exp. Med. 201, 41–47 (2005).
Kojo, S., Adachi, Y., Keino, H., Taniguchi, M. & Sumida, T. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 44, 1127–1138 (2001).
Linsen, L. et al. Peripheral blood but not synovial fluid natural killer T cells are biased towards a Th1-like phenotype in rheumatoid arthritis. Arthritis Res. Ther. 7, R493–R502 (2005).
Takahashi, T. et al. Cutting edge: analysis of human Vα24+CD8+ NK T cells activated by α-galactosylceramide-pulsed monocyte-derived dendritic cells. J. Immunol. 168, 3140–3144 (2002).
Li, X. et al. Invariant TCR rather than CD1d shapes the preferential activities of C-glycoside analogs against human versus murine invariant NKT Cells. J. Immunol. 183, 4415–4421 (2009).
Brossay, L. et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188, 1521–1528 (1998).
Tupin, E. et al. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc. Natl Acad. Sci. USA 105, 19863–19868 (2008).
Olson, C. M., Jr et al. Local production of IFN-γ by invariant NKT cells modulates acute Lyme carditis. J. Immunol. 182, 3728–3734 (2009).
Bharhani, M. S., Chiu, B., Na, K. S. & Inman, R. D. Activation of invariant NKT cells confers protection against Chlamydia trachomatis-induced arthritis. Int. Immunol. 21, 859–870 (2009).
Barral, D. C. & Brenner, M. B. CD1 antigen presentation: how it works. Nat. Rev. Immunol. 7, 929–941 (2007).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Morita, M. et al. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J. Med. Chem. 38, 2176–2187 (1995).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Drennan, M., Aspeslagh, S. & Elewaut, D. Invariant natural killer T cells in rheumatic disease: a joint dilemma. Nat Rev Rheumatol 6, 90–98 (2010). https://doi.org/10.1038/nrrheum.2009.261
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.261
This article is cited by
-
Altered thymic differentiation and modulation of arthritis by invariant NKT cells expressing mutant ZAP70
Nature Communications (2018)
-
IL-23 Responsive Innate-Like T Cells in Spondyloarthritis: the Less Frequent They Are, the More Vital They Appear
Current Rheumatology Reports (2015)