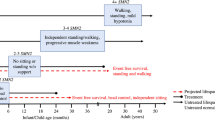
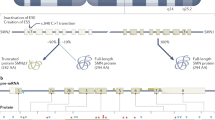
Key Points
-
The approval of nusinersen represents an important milestone for spinal muscular atrophy (SMA) research and treatment
-
Promising results from clinical trials indicate that several additional treatment options, such as gene therapy, could be available for patients with SMA in the near future
-
Preclinical research has highlighted the powerful potential of combinatorial targeting of both survival motor neuron protein (SMN)-dependent and SMN-independent pathways ('SMN-plus' therapy) to deliver maximal therapeutic benefits
-
The rapid emergence of many new therapeutic options for SMA raises major issues concerning the coordination of future clinical trials in this small population of patients
-
The development of therapies for SMA has the potential to offer important insights and tools that are applicable to patients with other neuromuscular and neurodegenerative conditions
Abstract
Spinal muscular atrophy (SMA) is a devastating motor neuron disease that predominantly affects children and represents the most common cause of hereditary infant mortality. The condition results from deleterious variants in SMN1, which lead to depletion of the survival motor neuron protein (SMN). Now, 20 years after the discovery of this genetic defect, a major milestone in SMA and motor neuron disease research has been reached with the approval of the first disease-modifying therapy for SMA by US and European authorities — the antisense oligonucleotide nusinersen. At the same time, promising data from early-stage clinical trials of SMN1 gene therapy have indicated that additional therapeutic options are likely to emerge for patients with SMA in the near future. However, the approval of nusinersen has generated a number of immediate and substantial medical, ethical and financial implications that have the potential to resonate beyond the specific treatment of SMA. Here, we provide an overview of the rapidly evolving therapeutic landscape for SMA, highlighting current achievements and future opportunities. We also discuss how these developments are providing important lessons for the emerging second generation of combinatorial ('SMN-plus') therapies that are likely to be required to generate robust treatments that are effective across a patient's lifespan.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lefebvre, S. et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155–165 (1995).
Verhaart, I. E. C. et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J. Rare Dis. 12, 124 (2017).
Lunn, M. R. & Wang, C. H. Spinal muscular atrophy. Lancet 371, 2120–2133 (2008).
Lorson, C. L., Hahnen, E., Androphy, E. J. & Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl Acad. Sci. USA 96, 6307–6311 (1999).
Monani, U. R. et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 8, 1177–1183 (1999).
Han, K. J. et al. Ubiquitin-specific protease 9x deubiquitinates and stabilizes the spinal muscular atrophy protein-survival motor neuron. J. Biol. Chem. 287, 43741–43752 (2012).
Lefebvre, S. et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 16, 265–269 (1997).
Battaglia, G., Princivalle, A., Forti, F., Lizier, C. & Zeviani, M. Expression of the SMN gene, the spinal muscular atrophy determining gene, in the mammalian central nervous system. Hum. Mol. Genet. 6, 1961–1971 (1997).
Burlet, P. et al. The distribution of SMN protein complex in human fetal tissues and its alteration in spinal muscular atrophy. Hum. Mol. Genet. 7, 1927–1933 (1998).
Coovert, D. D. et al. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 6, 1205–1214 (1997).
Lorson, M. A. & Lorson, C. L. SMN-inducing compounds for the treatment of spinal muscular atrophy. Future Med. Chem. 4, 2067–2084 (2012).
Singh, N. N., Howell, M. D., Androphy, E. J. & Singh, R. N. How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy. Gene Ther. 24, 520–526 (2017).
Schmutz, J. et al. The DNA sequence and comparative analysis of human chromosome 5. Nature 431, 268–274 (2004).
Monani, U. R. et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(−/−) mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 9, 333–339 (2000).
Wadman, R. I. et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0–4. J. Neurol. Neurosurg. Psychiatry 88, 365–367 (2017).
Crawford, T. O. et al. Evaluation of SMN protein, transcript, and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE 7, e33572 (2012).
Wadman, R. I. et al. A comparative study of SMN protein and mRNA in blood and fibroblasts in patients with spinal muscular atrophy and healthy controls. PLoS ONE 11, e0167087 (2016).
Hosseinibarkooie, S. et al. The power of human protective modifiers: PLS3 and CORO1C unravel impaired endocytosis in spinal muscular atrophy and rescue SMA phenotype. Am. J. Hum. Genet. 99, 647–665 (2016).
Oprea, G. E. et al. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science 320, 524–527 (2008).
Riessland, M. et al. Neurocalcin delta suppression protects against spinal muscular atrophy in humans and across species by restoring impaired endocytosis. Am. J. Hum. Genet. 100, 297–315 (2017).
Singh, R. N., Howell, M. D., Ottesen, E. W. & Singh, N. N. Diverse role of survival motor neuron protein. Biochim. Biophys. Acta 1860, 299–315 (2017).
Powis, R. A. & Gillingwater, T. H. Selective loss of alpha motor neurons with sparing of gamma motor neurons and spinal cord cholinergic neurons in a mouse model of spinal muscular atrophy. J. Anat. 228, 443–451 (2016).
Fletcher, E. V. et al. Reduced sensory synaptic excitation impairs motor neuron function via Kv2.1 in spinal muscular atrophy. Nat. Neurosci. 20, 905–916 (2017).
Hunter, G., Aghamaleky Sarvestany, A., Roche, S. L., Symes, R. C. & Gillingwater, T. H. SMN-dependent intrinsic defects in Schwann cells in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 23, 2235–2250 (2014).
Mentis, G. Z. et al. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron 69, 453–467 (2011).
Zhou, C., Feng, Z. & Ko, C. P. Defects in motoneuron-astrocyte interactions in spinal muscular atrophy. J. Neurosci. 36, 2543–2553 (2016).
Tisdale, S. & Pellizzoni, L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J. Neurosci. 35, 8691–8700 (2015).
Fischer, U., Liu, Q. & Dreyfuss, G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 90, 1023–1029 (1997).
Liu, Q., Fischer, U., Wang, F. & Dreyfuss, G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90, 1013–1021 (1997).
Pellizzoni, L., Yong, J. & Dreyfuss, G. Essential role for the SMN complex in the specificity of snRNP assembly. Science 298, 1775–1779 (2002).
Donlin-Asp, P. G. et al. The survival of motor neuron protein acts as a molecular chaperone for mRNP assembly. Cell Rep. 18, 1660–1673 (2017).
So, B. R. et al. A U1 snRNP-specific assembly pathway reveals the SMN complex as a versatile hub for RNP exchange. Nat. Struct. Mol. Biol. 23, 225–230 (2016).
Pellizzoni, L., Baccon, J., Charroux, B. & Dreyfuss, G. The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin and GAR1. Curr. Biol. 11, 1079–1088 (2001).
Piazzon, N. et al. Implication of the SMN complex in the biogenesis and steady state level of the signal recognition particle. Nucleic Acids Res. 41, 1255–1272 (2013).
Poole, A. R. & Hebert, M. D. SMN and coilin negatively regulate dyskerin association with telomerase RNA. Biol. Open 5, 726–735 (2016).
Baumer, D. et al. Alternative splicing events are a late feature of pathology in a mouse model of spinal muscular atrophy. PLoS Genet. 5, e1000773 (2009).
Zhang, Z. et al. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell 133, 585–600 (2008).
Boyd, P. & Gillingwater, T. H. in Spinal Muscular Atrophy (eds Sumner, C. J., Paushkin, S. & Ko, C. P.) 133–151 (Elsevier, 2016).
Cifuentes-Diaz, C. et al. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum. Mol. Genet. 11, 1439–1447 (2002).
Kariya, S. et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 17, 2552–2569 (2008).
Murray, L. M. et al. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 17, 949–962 (2008).
Kong, L. et al. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J. Neurosci. 29, 842–851 (2009).
Murray, L. M. et al. Pre-symptomatic development of lower motor neuron connectivity in a mouse model of severe spinal muscular atrophy. Hum. Mol. Genet. 19, 420–433 (2010).
Martinez-Hernandez, R. et al. Synaptic defects in type I spinal muscular atrophy in human development. J. Pathol. 229, 49–61 (2013).
Wadman, R. I., Vrancken, A. F., van den Berg, L. H. & van der Pol, W. L. Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Neurology 79, 2050–2055 (2012).
Kariya, S. et al. Requirement of enhanced survival motoneuron protein imposed during neuromuscular junction maturation. J. Clin. Invest. 124, 785–800 (2014).
Gillingwater, T. H. & Wishart, T. M. Mechanisms underlying synaptic vulnerability and degeneration in neurodegenerative disease. Neuropathol. Appl. Neurobiol. 39, 320–334 (2013).
Fuller, H. R. et al. Spinal muscular atrophy patient iPSC-derived motor neurons have reduced expression of proteins important in neuronal development. Front. Cell. Neurosci. 9, 506 (2015).
Ramser, J. et al. Rare missense and synonymous variants in UBE1 are associated with X-linked infantile spinal muscular atrophy. Am. J. Hum. Genet. 82, 188–193 (2008).
Wishart, T. M. et al. Dysregulation of ubiquitin homeostasis and beta-catenin signaling promote spinal muscular atrophy. J. Clin. Invest. 124, 1821–1834 (2014).
Powis, R. A. et al. Systemic restoration of UBA1 ameliorates disease in spinal muscular atrophy. JCI Insight 1, e87908 (2016).
Berger, A. et al. Severe depletion of mitochondrial DNA in spinal muscular atrophy. Acta Neuropathol. 105, 245–251 (2003).
Ripolone, M. et al. Impaired muscle mitochondrial biogenesis and myogenesis in spinal muscular atrophy. JAMA Neurol. 72, 666–675 (2015).
Acsadi, G. et al. Mitochondrial dysfunction in a neural cell model of spinal muscular atrophy. J. Neurosci. Res. 87, 2748–2756 (2009).
Miller, N., Shi, H., Zelikovich, A. S. & Ma, Y. C. Motor neuron mitochondrial dysfunction in spinal muscular atrophy. Hum. Mol. Genet. 25, 3395–3406 (2016).
Xu, C. C., Denton, K. R., Wang, Z. B., Zhang, X. & Li, X. J. Abnormal mitochondrial transport and morphology as early pathological changes in human models of spinal muscular atrophy. Dis. Model. Mech. 9, 39–49 (2016).
Boyd, P. J. et al. Bioenergetic status modulates motor neuron vulnerability and pathogenesis in a zebrafish model of spinal muscular atrophy. PLoS Genet. 13, e1006744 (2017).
Hosseinibarkooie, S., Schneider, S. & Wirth, B. Advances in understanding the role of disease-associated proteins in spinal muscular atrophy. Expert Rev. Proteom. 14, 581–592 (2017).
Bowerman, M., Shafey, D. & Kothary, R. Smn depletion alters profilin II expression and leads to upregulation of the RhoA/ROCK pathway and defects in neuronal integrity. J. Mol. Neurosci. 32, 120–131 (2007).
Nolle, A. et al. The spinal muscular atrophy disease protein SMN is linked to the Rho-kinase pathway via profilin. Hum. Mol. Genet. 20, 4865–4878 (2011).
Bowerman, M., Beauvais, A., Anderson, C. L. & Kothary, R. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum. Mol. Genet. 19, 1468–1478 (2010).
Bowerman, M., Murray, L. M., Boyer, J. G., Anderson, C. L. & Kothary, R. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC Med. 10, 24 (2012).
Kaifer, K. A. et al. Plastin-3 extends survival and reduces severity in mouse models of spinal muscular atrophy. JCI Insight 2, e89970 (2017).
Holt, C. E. & Schuman, E. M. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 80, 648–657 (2013).
Kapur, M., Monaghan, C. E. & Ackerman, S. L. Regulation of mRNA translation in neurons — a matter of life and death. Neuron 96, 616–637 (2017).
Akten, B. et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc. Natl Acad. Sci. USA 108, 10337–10342 (2011).
Fallini, C., Donlin-Asp, P. G., Rouanet, J. P., Bassell, G. J. & Rossoll, W. Deficiency of the survival of motor neuron protein impairs mRNA localization and local translation in the growth cone of motor neurons. J. Neurosci. 36, 3811–3820 (2016).
Fallini, C. et al. The survival of motor neuron (SMN) protein interacts with the mRNA-binding protein HuD and regulates localization of poly(A) mRNA in primary motor neuron axons. J. Neurosci. 31, 3914–3925 (2011).
Hubers, L. et al. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy-like neuronal defects. Hum. Mol. Genet. 20, 553–579 (2011).
Rossoll, W. et al. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J. Cell Biol. 163, 801–812 (2003).
Kye, M. J. et al. SMN regulates axonal local translation via miR-183/mTOR pathway. Hum. Mol. Genet. 23, 6318–6331 (2014).
Sanchez, G. et al. A novel role for CARM1 in promoting nonsense-mediated mRNA decay: potential implications for spinal muscular atrophy. Nucleic Acids Res. 44, 2661–2676 (2016).
Sanchez, G. et al. A novel function for the survival motoneuron protein as a translational regulator. Hum. Mol. Genet. 22, 668–684 (2013).
Bernabo, P. et al. In vivo translatome profiling in spinal muscular atrophy reveals a role for SMN protein in ribosome biology. Cell Rep. 21, 953–965 (2017).
Hua, Y. et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478, 123–126 (2011).
Zhou, H. et al. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene Ther. 24, 331–342 (2013).
Chiriboga, C. A. et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 86, 890–897 (2016).
Finkel, R. S. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388, 3017–3026 (2016).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Hammond, S. M. et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc. Natl Acad. Sci. USA 113, 10962–10967 (2016).
Naryshkin, N. A. et al. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 345, 688–693 (2014).
Abera, M. B. et al. ML372 blocks SMN ubiquitination and improves spinal muscular atrophy pathology in mice. JCI Insight 1, e88427 (2016).
Avila, A. M. et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Invest. 117, 659–671 (2007).
Farooq, F. et al. Prolactin increases SMN expression and survival in a mouse model of severe spinal muscular atrophy via the STAT5 pathway. J. Clin. Invest. 121, 3042–3050 (2011).
Kwon, D. Y. et al. The E3 ubiquitin ligase mind bomb 1 ubiquitinates and promotes the degradation of survival of motor neuron protein. Mol. Biol. Cell 24, 1863–1871 (2013).
Riessland, M. et al. SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Hum. Mol. Genet. 19, 1492–1506 (2010).
Kissel, J. T. et al. SMA CARNIVAL TRIAL PART II: a prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PLoS ONE 6, e21296 (2011).
Renusch, S. R. et al. Spinal muscular atrophy biomarker measurements from blood samples in a clinical trial of valproic acid in ambulatory adults. J. Neuromuscul. Dis. 2, 119–130 (2015).
Krosschell, K. J. et al. Clinical trial of L-carnitine and valproic acid in spinal muscular atrophy type I. Muscle Nerve https://doi.org/10.1002/mus.25776 (2017).
Dominguez, E. et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet. 20, 681–693 (2011).
Foust, K. D. et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 28, 271–274 (2010).
Passini, M. A. et al. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J. Clin. Invest. 120, 1253–1264 (2010).
Valori, C. F. et al. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci. Transl Med. 2, 35ra42 (2010).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Bertini, E. et al. Safety and efficacy of olesoxime in patients with type 2 or non-ambulatory type 3 spinal muscular atrophy: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 16, 513–522 (2017).
Hwee, D. T. et al. The small-molecule fast skeletal troponin activator, CK-2127107, improves exercise tolerance in a rat model of heart failure. J. Pharmacol. Exp. Ther. 353, 159–168 (2015).
Christie-Brown, V., Mitchell, J. & Talbot, K. The SMA Trust: the role of a disease-focused research charity in developing treatments for SMA. Gene Ther. 24, 544–546 (2017).
Gillingwater, T. H. Dawn of a new therapeutic era for spinal muscular atrophy. Lancet 388, 2964–2965 (2016).
Aartsma-Rus, A. FDA approval of nusinersen for spinal muscular atrophy makes 2016 the year of splice modulating oligonucleotides. Nucleic Acid. Ther. 27, 67–69 (2017).
van der Ploeg, A. T. The dilemma of two innovative therapies for spinal muscular atrophy. N. Engl. J. Med. 377, 1786–1787 (2017).
Hache, M. et al. Intrathecal injections in children with spinal muscular atrophy: nusinersen clinical trial experience. J. Child Neurol. 31, 899–906 (2016).
Farrar, M. A. et al. Emerging therapies and challenges in spinal muscular atrophy. Ann. Neurol. 81, 355–368 (2017).
Dobrowolski, S. F. et al. Newborn screening for spinal muscular atrophy by calibrated short-amplicon melt profiling. Clin. Chem. 58, 1033–1039 (2012).
Taylor, J. L. et al. Newborn blood spot screening test using multiplexed real-time PCR to simultaneously screen for spinal muscular atrophy and severe combined immunodeficiency. Clin. Chem. 61, 412–419 (2015).
Lin, P. J., Yeh, W. S. & Neumann, P. J. Willingness to pay for a newborn screening test for spinal muscular atrophy. Pediatr. Neurol. 66, 69–75 (2017).
Bevan, A. K. et al. Early heart failure in the SMNDelta7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum. Mol. Genet. 19, 3895–3905 (2010).
Deguise, M. O. et al. Immune dysregulation may contribute to disease pathogenesis in spinal muscular atrophy mice. Hum. Mol. Genet. 26, 801–819 (2017).
Somers, E. et al. Vascular defects and spinal cord hypoxia in spinal muscular atrophy. Ann. Neurol. 79, 217–230 (2016).
Szunyogova, E. et al. Survival Motor Neuron (SMN) protein is required for normal mouse liver development. Sci. Rep. 6, 34635 (2016).
Thomson, A. K. et al. Survival of motor neurone protein is required for normal postnatal development of the spleen. J. Anat. 230, 337–346 (2017).
Wijngaarde, C. A. et al. Cardiac pathology in spinal muscular atrophy: a systematic review. Orphanet J. Rare Dis. 12, 67 (2017).
Hamilton, G. & Gillingwater, T. H. Spinal muscular atrophy: going beyond the motor neuron. Trends Mol. Med. 19, 40–50 (2013).
McGraw, S. et al. A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. 17, 68 (2017).
King, N. M. P. & Bishop, C. E. New treatments for serious conditions: ethical implications. Gene Ther. 24, 534–538 (2017).
Friedmann, T. Gene therapy for spinomuscular atrophy: a biomedical advance, a missed opportunity for more equitable drug pricing. Gene Ther. 24, 503–505 (2017).
Young, K. E., Soussi, I., Hemels, M. & Toumi, M. A comparative study of orphan drug prices in Europe. J. Mark. Access Health Policy 5, 1297886 (2017).
Gammie, T., Lu, C. Y. & Babar, Z. U. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLoS ONE 10, e0140002 (2015).
Penington, R. & Stubbings, J. A. Evaluation of specialty drug price trends using data from retrospective pharmacy sales transactions. J. Manag. Care Spec. Pharm. 22, 1010–1017 (2016).
Qian, Y. et al. Understanding the experiences and needs of individuals with Spinal Muscular Atrophy and their parents: a qualitative study. BMC Neurol. 15, 217 (2015).
Armstrong, E. P. et al. The economic burden of spinal muscular atrophy. J. Med. Econ. 19, 822–826 (2016).
Gillingwater, T. H. Counting the cost of spinal muscular atrophy. J. Med. Econ. 19, 827–828 (2016).
Klug, C. et al. Disease burden of spinal muscular atrophy in Germany. Orphanet J. Rare Dis. 11, 58 (2016).
Montes, J. et al. Spinal muscular atrophy functional composite score: A functional measure in spinal muscular atrophy. Muscle Nerve 52, 942–947 (2015).
Pera, M. C. et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol. 17, 39 (2017).
Ramsey, D. et al. Revised Hammersmith Scale for spinal muscular atrophy: A SMA specific clinical outcome assessment tool. PLoS One 12, e0172346 (2017).
Tizzano, E. F. & Finkel, R. S. Spinal muscular atrophy: a changing phenotype beyond the clinical trials. Neuromuscul. Disord. 27, 883–889 (2017).
Groen, E. J. & Gillingwater, T. H. UBA1: at the crossroads of ubiquitin homeostasis and neurodegeneration. Trends Mol. Med. 21, 622–632 (2015).
Groen, E. J. et al. ALS-associated mutations in FUS disrupt the axonal distribution and function of SMN. Hum. Mol. Genet. 22, 3690–3704 (2013).
Perera, N. D. et al. Enhancing survival motor neuron expression extends lifespan and attenuates neurodegeneration in mutant TDP-43 mice. Hum. Mol. Genet. 25, 4080–4093 (2016).
Rodriguez-Muela, N. et al. Single-cell analysis of SMN reveals its broader role in neuromuscular disease. Cell Rep. 18, 1484–1498 (2017).
Sun, S. et al. ALS-causative mutations in FUS/TLS confer gain and loss of function by altered association with SMN and U1-snRNP. Nat. Commun. 6, 6171 (2015).
Yamazaki, T. et al. FUS-SMN protein interactions link the motor neuron diseases ALS and SMA. Cell Rep. 2, 799–806 (2012).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research, discussion, writing and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.H.G. and K.T. serve as Chair of the Scientific and Clinical Advisory Board and Trustee of the SMA Trust, respectively.
Rights and permissions
About this article
Cite this article
Groen, E., Talbot, K. & Gillingwater, T. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat Rev Neurol 14, 214–224 (2018). https://doi.org/10.1038/nrneurol.2018.4
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2018.4
This article is cited by
-
Optimized MLPA workflow for spinal muscular atrophy diagnosis: identification of a novel variant, NC_000005.10:g.(70919941_70927324)del in isolated exon 1 of SMN1 gene through long-range PCR
BMC Neurology (2024)
-
Programmable synthetic receptors: the next-generation of cell and gene therapies
Signal Transduction and Targeted Therapy (2024)
-
CircHIPK3 regulates fatty acid metabolism through miR-637/FASN axis to promote esophageal squamous cell carcinoma
Cell Death Discovery (2024)
-
Assessment of motor function and nutritional status in children with spinal muscular atrophy treated with nusinersen after loading period in Western China: a retrospective study
BMC Neurology (2023)
-
Optimization of base editors for the functional correction of SMN2 as a treatment for spinal muscular atrophy
Nature Biomedical Engineering (2023)