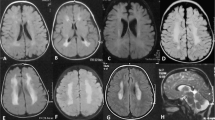
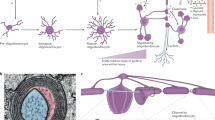
Key Points
-
Leukodystrophies are a heterogeneous group of inherited disorders with highly variable clinical manifestations and pathogenetic background
-
Leukodystrophies are characterized by primary glial cell and myelin sheath pathology of variable aetiology; secondary axonal pathology can emerge as the disease progresses
-
Around 20 distinct disorders are currently defined as adulthood leukodystrophies; additional involvement of grey matter structures or non-cerebral organs distinguishes these conditions from other genetic leukoencephalopathies
-
Increasing numbers of individual leukodystrophies are being treated using metabolic treatment strategies, enzyme replacement or cell-based options such as allogeneic haematopoietic stem cell transplantation and gene therapy
Abstract
The leukodystrophies are a group of inherited white matter disorders with a heterogeneous genetic background, considerable phenotypic variability and disease onset at all ages. This Review focuses on leukodystrophies with major prevalence or primary onset in adulthood. We summarize 20 leukodystrophies with adult presentations, providing information on the underlying genetic mutations and on biochemical assays that aid diagnosis, where available. Definitions, clinical characteristics, age of onset, MRI findings and treatment options are all described, providing a comprehensive overview of the current knowledge of the various adulthood leukodystrophies. We highlight the distinction between adult-onset leukodystrophies and other inherited disorders with white matter involvement, and we propose a diagnostic pathway for timely recognition of adulthood leukodystrophies in a routine clinical setting. In addition, we provide detailed clinical information on selected adult-onset leukodystrophies, including X-linked adrenoleukodystrophy, metachromatic leukodystrophy, cerebrotendinous xanthomatosis, hereditary diffuse leukoencephalopathy with axonal spheroids, autosomal dominant adult-onset demyelinating leukodystrophy, adult polyglucosan body disease, and leukoencephalopathy with vanishing white matter. Ultimately, this Review aims to provide helpful suggestions to identify treatable adulthood leukodystrophies at an early stage in the disease course.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vanderver, A. et al. Case definition and classification of leukodystrophies and leukoencephalopathies. Mol. Genet. Metab. 114, 494–500 (2015).
Parikh, S. et al. A clinical approach to the diagnosis of patients with leukodystrophies and genetic leukoencephelopathies. Mol. Genet. Metab. 114, 501–515 (2015).
Ahmed, R. M. et al. A practical approach to diagnosing adult onset leukodystrophies. J. Neurol. Neurosurg. Psychiatry 85, 770–781 (2014).
Kohlschutter, A. & Eichler, F. Childhood leukodystrophies: a clinical perspective. Expert Rev. Neurother. 11, 1485–1496 (2011).
Van der Knaap, M. S. & Buguani, M. Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol. 134, 351–182 (2017).
Schiffmann, R. & van der Knaap, M. S. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 72, 750–759 (2009).
Pouwels, P. J. et al. Hypomyelinating leukodystrophies: translational research progress and prospects. Ann. Neurol. 76, 15–19 (2014).
Steenweg, M. E. et al. Magnetic resonance imaging pattern recognition in hypomyelinating disorders. Brain 133, 2971–2982 (2010).
Di Rocco, M., Doria-Lamba, L. & Caruso, U. Monozygotic twins with X-linked adrenoleukodystrophy and different phenotypes. Ann. Neurol. 50, 424 (2001).
Moser, H. W. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 120, 1485–1508 (1997).
Hellmann, M. A. et al. Frequent misdiagnosis of adult polyglucosan body disease. J. Neurol. 262, 2346–2351 (2015).
von Figura, K., Steckel, F., Conary, J., Hasilik, A. & Shaw, E. Heterogeneity in late-onset metachromatic leukodystrophy. Effect of inhibitors of cysteine proteinases. Am. J. Hum. Genet. 39, 371–382 (1986).
Lopez-Hernandez, T. et al. Mutant GlialCAM causes megalencephalic leukoencephalopathy with subcortical cysts, benign familial macrocephaly, and macrocephaly with retardation and autism. Am. J. Hum. Genet. 88, 422–432 (2011).
Schmahmann, J. D., Smith, E. E., Eichler, F. S. & Filley, C. M. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. NY Acad. Sci. 1142, 266–309 (2008).
Adang, L. A. et al. Revised consensus statement on the preventive and symptomatic care of patients with leukodystrophies. Mol. Genet. Metab. 122, 18–32 (2017).
Heim, P. et al. Leukodystrophy incidence in Germany. Am. J. Med. Genet. 71, 475–478 (1997).
Bonkowsky, J. L. et al. The burden of inherited leukodystrophies in children. Neurology 75, 718–725 (2010).
Bezman, L. et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 49, 512–517 (2001).
Vanderver, A., Hussey, H., Schmidt, J. L., Pastor, W. & Hoffman, H. J. Relative incidence of inherited white matter disorders in childhood to acquired pediatric demyelinating disorders. Semin. Pediatr. Neurol. 19, 219–223 (2012).
Ayrignac, X. et al. Adult-onset genetic leukoencephalopathies: a MRI pattern-based approach in a comprehensive study of 154 patients. Brain 138, 284–292 (2015).
van der Knaap, M. S., Breiter, S. N., Naidu, S., Hart, A. A. & Valk, J. Defining and categorizing leukoencephalopathies of unknown origin: MR imaging approach. Radiology 213, 121–133 (1999).
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E. & Bousser, M. G. CADASIL Lancet Neurol. 8, 643–653 (2009).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011).
Loes, D. J. et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am. J. Neuroradiol. 15, 1761–1766 (1994).
Vanderver, A. et al. Whole exome sequencing in patients with white matter abnormalities. Ann. Neurol. 79, 1031–1037 (2016).
Kevelam, S. H. et al. Update on leukodystrophies: a historical perspective and adapted definition. Neuropediatrics 47, 349–354 (2016).
Mosser, J. et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993).
Moser, H. W. et al. Adrenoleukodystrophy: elevated C26 fatty acid in cultured skin fibroblasts. Ann. Neurol. 7, 542–549 (1980).
Berger, J., Forss-Petter, S. & Eichler, F. S. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 98, 135–142 (2014).
Kemp, S., Huffnagel, I. C., Linthorst, G. E., Wanders, R. J. & Engelen, M. Adrenoleukodystrophy — neuroendocrine pathogenesis and redefinition of natural history. Nat. Rev. Endocrinol. 12, 606–615 (2016).
Peters, C. et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood 104, 881–888 (2004).
Kühl, J. S. et al. Long-term outcomes of allogeneic haematopoietic stem cell transplantation for adult cerebral X-linked adrenoleukodystrophy. Brain 140, 953–966 (2017).
van Geel, B. M. et al. Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. J. Inherit. Metab. Dis. 38, 359–361 (2015).
Cartier, N. et al. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzymol. 507, 187–198 (2012).
Eichler, F. et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 377, 1630–1638 (2017).
Engelen, M. Optimizing treatment for cerebral adrenoleukodystrophy in the era of gene therapy. N. Engl. J. Med. 377, 1682–1684 (2017).
Semmler, A., Kohler, W., Jung, H. H., Weller, M. & Linnebank, M. Therapy of X-linked adrenoleukodystrophy. Expert Rev. Neurother. 8, 1367–1379 (2008).
Sedel, F., Bernard, D., Mock, D. M. & Tourbah, A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology 110, 644–653 (2016).
Morato, L. et al. Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy. Brain 136, 2432–2443 (2013).
Hubbard, W. C. et al. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography-tandem mass spectrometric (LC-MS/MS) method. Mol. Genet. Metab. 97, 212–220 (2009).
Theda, C. et al. Newborn screening for X-linked adrenoleukodystrophy: further evidence high throughput screening is feasible. Mol. Genet. Metab. 111, 55–57 (2014).
Baumann, N. et al. Adult forms of metachromatic leukodystrophy: clinical and biochemical approach. Dev. Neurosci. 13, 211–215 (1991).
Betts, T. A., Smith, W. T. & Kelly, R. E. Adult metachromatic leukodystrophy (sulphatide lipidosis) simulating acute schizophrenia. Report of a case. Neurology 18, 1140–1142 (1968).
Gieselmann, V., Fluharty, A. L., Tonnesen, T. & Von Figura, K. Mutations in the arylsulfatase A pseudodeficiency allele causing metachromatic leukodystrophy. Am. J. Hum. Genet. 49, 407–413 (1991).
van Rappard, D. F. et al. Gallbladder and the risk of polyps and carcinoma in metachromatic leukodystrophy. Neurology 87, 103–111 (2016).
Cesani, M. et al. Mutation update of ARSA and PSAP genes causing metachromatic leukodystrophy. Hum. Mutat. 37, 16–27 (2016).
Matthes, F. et al. Efficacy of enzyme replacement therapy in an aggravated mouse model of metachromatic leukodystrophy declines with age. Hum. Mol. Genet. 21, 2599–2609 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01510028?term=NCT01510028&rank=1 (2017).
Krivit, W., Peters, C. & Shapiro, E. G. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux–Lamy, and Sly syndromes, and Gaucher disease type III. Curr. Opin. Neurol. 12, 167–176 (1999).
Sevin, C., Aubourg, P. & Cartier, N. Enzyme, cell and gene-based therapies for metachromatic leukodystrophy. J. Inherit. Metab. Dis. 30, 175–183 (2007).
Bredius, R. G. et al. Early marrow transplantation in a pre-symptomatic neonate with late infantile metachromatic leukodystrophy does not halt disease progression. Bone Marrow Transplant. 39, 309–310 (2007).
van Egmond, M. E. et al. Improvement of white matter changes on neuroimaging modalities after stem cell transplant in metachromatic leukodystrophy. JAMA Neurol. 70, 779–782 (2013).
van Rappard, D. F., Boelens, J. J. & Wolf, N. I. Metachromatic leukodystrophy: disease spectrum and approaches for treatment. Best Pract. Res. Clin. Endocrinol. Metab. 29, 261–273 (2015).
Boucher, A. A. et al. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: the largest single-institution cohort report. Orphanet J. Rare Dis. 10, 94 (2015).
Groeschel, S. et al. Long-term Outcome of allogeneic hematopoietic stem cell transplantation in patients with juvenile metachromatic leukodystrophy compared with nontransplanted control patients. JAMA Neurol. 73, 1133–1140 (2016).
Sessa, M. et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet 388, 476–487 (2016).
Dotti, M. T., Rufa, A. & Federico, A. Cerebrotendinous xanthomatosis: heterogeneity of clinical phenotype with evidence of previously undescribed ophthalmological findings. J. Inherit. Metab. Dis. 24, 696–706 (2001).
Verrips, A. et al. Spinal xanthomatosis: a variant of cerebrotendinous xanthomatosis. Brain 122, 1589–1595 (1999).
Bjorkhem, I. et al. Role of the 26-hydroxylase in the biosynthesis of bile acids in the normal state and in cerebrotendinous xanthomatosis. An in vivo study. J. Clin. Invest. 71, 142–148 (1983).
Koopman, B. J. et al. Cerebrotendinous xanthomatosis: a review of biochemical findings of the patient population in The Netherlands. J. Inherit. Metab. Dis. 11, 56–75 (1988).
Federico, A., Dotti, M. T. & Gallus, G. N. Cerebrotendinous xanthomatosis. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1409/ (updated 14 April 2016).
Nie, S., Chen, G., Cao, X. & Zhang, Y. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 9, 179 (2014).
Freeman, S. H. et al. Adult onset leukodystrophy with neuroaxonal spheroids: clinical, neuroimaging and neuropathologic observations. Brain Pathol. 19, 39–47 (2009).
Sundal, C. et al. Parkinsonian features in hereditary diffuse leukoencephalopathy with spheroids (HDLS) and CSF1R mutations. Parkinsonism Relat. Disord. 19, 869–877 (2013).
Rademakers, R. et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 44, 200–205 (2011).
Sundal, C. et al. Hereditary diffuse leukoencephalopathy with spheroids with phenotype of primary progressive multiple sclerosis. Eur. J. Neurol. 22, 328–333 (2015).
Eichler, F. S. et al. CSF1R mosaicism in a family with hereditary diffuse leukoencephalopathy with spheroids. Brain 139, 1666–1672 (2016).
Padiath, Q. S. et al. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat. Genet. 38, 1114–1123 (2006).
Schuster, J. et al. Genomic duplications mediate overexpression of lamin B1 in adult-onset autosomal dominant leukodystrophy (ADLD) with autonomic symptoms. Neurogenetics 12, 65–72 (2011).
Eldridge, R. et al. Hereditary adult-onset leukodystrophy simulating chronic progressive multiple sclerosis. N. Engl. J. Med. 311, 948–953 (1984).
van der Knaap, M. S. et al. A new leukoencephalopathy with vanishing white matter. Neurology 48, 845–855 (1997).
Schiffmann, R. et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann. Neurol. 35, 331–340 (1994).
van der Knaap, M. S., Pronk, J. C. & Scheper, G. C. Vanishing white matter disease. Lancet Neurol. 5, 413–423 (2006).
Labauge, P. et al. Natural history of adult-onset eIF2B-related disorders: a multi-centric survey of 16 cases. Brain 132, 2161–2169 (2009).
Fogli, A. et al. Ovarian failure related to eukaryotic initiation factor 2B mutations. Am. J. Hum. Genet. 72, 1544–1550 (2003).
Mochel, F. et al. Adult polyglucosan body disease: natural history and key magnetic resonance imaging findings. Ann. Neurol. 72, 433–441 (2012).
Klein, C. J. Adult polyglucosan body disease. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK5300/ (updated 2 April 2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00947960?term=NCT00947960&rank=1 (2016).
Roe, C. R., Bottiglieri, T., Wallace, M., Arning, E. & Martin, A. Adult polyglucosan body disease (APBD): anaplerotic diet therapy (triheptanoin) and demonstration of defective methylation pathways. Mol. Genet. Metab. 101, 246–252 (2010).
Helman, G. et al. Disease specific therapies in leukodystrophies and leukoencephalopathies. Mol. Genet. Metab. 114, 527–536 (2015).
van der Knaap, M. S. et al. New syndrome characterized by hypomyelination with atrophy of the basal ganglia and cerebellum. AJNR Am. J. Neuroradiol. 23, 1466–1474 (2002).
Garbern, J. Y. et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125, 551–561 (2002).
Uhlenberg, B. et al. Mutations in the gene encoding gap junction protein α12 (connexin 46.6) cause Pelizaeus–Merzbacher-like disease. Am. J. Hum. Genet. 75, 251–260 (2004).
Wolf, N. I. et al. Clinical spectrum of 4H leukodystrophy caused by POLR3A and POLR3B mutations. Neurology 83, 1898–1905 (2014).
Dallabona, C. et al. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology 82, 2063–2071 (2014).
Balbi, P. et al. The clinical spectrum of late-onset Alexander disease: a systematic literature review. J. Neurol. 257, 1955–1962 (2010).
Jeworutzki, E. et al. GlialCAM, a protein defective in a leukodystrophy, serves as a ClC-2 Cl− channel auxiliary subunit. Neuron 73, 951–961 (2012).
Depienne, C. et al. Brain white matter oedema due to ClC-2 chloride channel deficiency: an observational analytical study. Lancet Neurol. 12, 659–668 (2013).
Edvardson, S. et al. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am. J. Hum. Genet. 83, 643–648 (2008).
Graziano, A. C. & Cardile, V. History, genetic, and recent advances on Krabbe disease. Gene 555, 2–13 (2015).
van Berge, L. et al. Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: clinical and genetic characterization and target for therapy. Brain 137, 1019–1029 (2014).
Lossos, A. et al. Phenotypic variability among adult siblings with Sjogren–Larsson syndrome. Arch. Neurol. 63, 278–280 (2006).
Onodera, O., Nozaki, H. & Fukutake, T. CARASIL. GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK32533/ (updated 11 September 2014).
Choi, J. C. Genetics of cerebral small vessel disease. J. Stroke 17, 7–16 (2015).
Bottcher, T. et al. Fabry disease — underestimated in the differential diagnosis of multiple sclerosis? PLoS ONE 8, e71894 (2013).
Kolar, G. R. et al. Neuropathology and genetics of cerebroretinal vasculopathies. Brain Pathol. 24, 510–518 (2014).
Paloneva, J., Autti, T., Hakola, P. & Haltia, M. J. Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL). GeneReviews http://www.ncbi.nlm.nih.gov/books/NBK1197/ (updated 12 March 2015).
Girard, J. M., Turnbull, J., Ramachandran, N. & Minassian, B. A. Progressive myoclonus epilepsy. Handb. Clin. Neurol. 113, 1731–1736 (2013).
Hagerman, P. J. & Hagerman, R. J. Fragile X-associated tremor/ataxia syndrome. Ann. NY Acad. Sci. 1338, 58–70 (2015).
Aronson, N. N. Jr. Aspartylglycosaminuria: biochemistry and molecular biology. Biochim. Biophys. Acta 1455, 139–154 (1999).
Baumgartner, M. R. et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 9, 130 (2014).
Borgwardt, L. et al. Alpha-mannosidosis: correlation between phenotype, genotype and mutant MAN2B1 subcellular localisation. Orphanet J. Rare Dis. 10, 70 (2015).
Sedel, F. et al. Psychiatric manifestations revealing inborn errors of metabolism in adolescents and adults. J. Inherit. Metab. Dis. 30, 631–641 (2007).
Verheijen, F. W. et al. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nat. Genet. 23, 462–465 (1999).
Rubio-Agusti, I. et al. Movement disorders in adult patients with classical galactosemia. Mov. Disord. 28, 804–810 (2013).
Yoshida, K. et al. GM1 gangliosidosis in adults: clinical and molecular analysis of 16 Japanese patients. Ann. Neurol. 31, 328–332 (1992).
Argov, Z. & Navon, R. Clinical and genetic variations in the syndrome of adult GM2 gangliosidosis resulting from hexosaminidase A deficiency. Ann. Neurol. 16, 14–20 (1984).
Wilcken, B. Leukoencephalopathies associated with disorders of cobalamin and folate metabolism. Semin. Neurol. 32, 68–74 (2012).
Lossos, A. et al. Severe methylenetetrahydrofolate reductase deficiency: clinical clues to a potentially treatable cause of adult-onset hereditary spastic paraplegia. JAMA Neurol. 71, 901–904 (2014).
Skovby, F., Gaustadnes, M. & Mudd, S. H. A revisit to the natural history of homocystinuria due to cystathionine beta-synthase deficiency. Mol. Genet. Metab. 99, 1–3 (2010).
Ding, X. Q. et al. MRI abnormalities in normal-appearing brain tissue of treated adult PKU patients. J. Magn. Reson. Imag. 27, 998–1004 (2008).
Marcel, C. et al. L-2-hydroxyglutaric aciduria diagnosed in a young adult with progressive cerebellar ataxia and facial dyskinesia. Rev. Neurol. (Paris) 168, 187–191 (2012).
Reimao, S. et al. 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: initial presentation in a young adult. J. Inherit. Metab. Dis. 32 (Suppl. 1), 49–52 (2009).
Bandmann, O., Weiss, K. H. & Kaler, S. G. Wilson's disease and other neurological copper disorders. Lancet Neurol. 14, 103–113 (2015).
Acknowledgements
The authors thank the patients and their families for their long-lasting confidence and abundance of patience while waiting for relief from the disease burden and new treatment options. The authors also acknowledge many years of constant support and inspiration from family organizations such as the European Leukodystrophy Association, the Myelin Project, the Adrenoleukodystrophy (ALD) Charity, the Stop ALD Foundation and the United Leukodystrophy Foundation. W.K. received funding from the German Ministry of Education and Research as part of the German LEUKONET Network.
Author information
Authors and Affiliations
Contributions
W.K. researched data for the article and wrote the text. All three authors made substantial contributions to discussions of the content, and W.K. and A.V. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (table)
Inherited childhood leukodystrophies (DOC 36 kb)
Rights and permissions
About this article
Cite this article
Köhler, W., Curiel, J. & Vanderver, A. Adulthood leukodystrophies. Nat Rev Neurol 14, 94–105 (2018). https://doi.org/10.1038/nrneurol.2017.175
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.175
This article is cited by
-
Therapie der X-chromosomalen Adrenoleukodystrophie
DGNeurologie (2024)
-
Endogenous Sox8 is a critical factor for timely remyelination and oligodendroglial cell repletion in the cuprizone model
Scientific Reports (2023)
-
Early leukoencephalopathy during daratumumab treatment in a patient with multiple myeloma
Annals of Hematology (2023)
-
High genetic heterogeneity of leukodystrophies in Iranian children: the first report of Iranian Leukodystrophy Registry
neurogenetics (2023)
-
A case of primary optic pathway demyelination caused by oncocytic oligodendrogliopathy of unknown origin
Acta Neuropathologica Communications (2022)