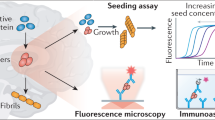
Key Points
-
Alzheimer disease belongs to the group of proteinopathies; it is characterized by deposition of the peptide amyloid-β (Aβ) as amyloid plaques and of the protein tau as neurofibrillary tangles
-
Aβ neurotoxicity is attributable to specific types of Aβ, generated as a consequence of proteolytic cleavage and post-translational modifications, which are susceptible to aggregation into different assembly states
-
Similar to Aβ, tau exists as multiple brain isoforms that undergo aggregation and is subject to a host of post-translational modifications, including phosphorylation and acetylation
-
Impairment of multiple cellular functions by Aβ and tau has been demonstrated in cellular and transgenic models, and crosstalk between these molecules has been demonstrated, particularly at the synapse
-
Assessment of Aβ and tau pathology is being facilitated by increasingly sensitive methods, which have clinical relevance in diagnosis and in the validation of therapeutic interventions in disease
-
A prerequisite for personalized medicine is the identification of distinct Aβ and tau species that are suitable for use as biomarkers in cerebrospinal fluid, blood, urine and saliva
Abstract
Most neurodegenerative diseases are proteinopathies, which are characterized by the aggregation of misfolded proteins. Although many proteins have an intrinsic propensity to aggregate, particularly when cellular clearance systems start to fail in the context of ageing, only a few form fibrillar aggregates. In Alzheimer disease, the peptide amyloid-β (Aβ) and the protein tau aggregate to form plaques and tangles, respectively, which comprise the histopathological hallmarks of this disease. This Review discusses the complexity of Aβ biogenesis, trafficking, post-translational modifications and aggregation states. Tau and its various isoforms, which are subject to a vast array of post-translational modifications, are also explored. The methodological advances that revealed this complexity are described. Finally, the toxic effects of distinct species of tau and Aβ are discussed, as well as the concept of protein 'strains', and how this knowledge can facilitate the development of early disease biomarkers for stratifying patients and validating new therapies. By targeting distinct species of Aβ and tau for therapeutic intervention, the way might be paved for personalized medicine and more-targeted treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brookmeyer, R., Johnson, E., Ziegler-Graham, K. & Arrighi, H. M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 3, 186–191 (2007).
Ballard, C. et al. Alzheimer's disease. Lancet 377, 1019–1031 (2011).
Jahn, T. R. et al. The common architecture of cross-β amyloid. J. Mol. Biol. 395, 717–727 (2010).
Ross, C. A. & Poirier, M. A. Protein aggregation and neurodegenerative disease. Nat. Med. 10 (Suppl.), S10–S17 (2004).
Cacace, R., Sleegers, K. & Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer's disease revisited. Alzheimers Dement. 12, 733–748 (2016).
Götz, J. & Ittner, L. M. Animal models of Alzheimer's disease and frontotemporal dementia. Nat. Rev. Neurosci. 9, 532–544 (2008).
Shariati, S. A. & De Strooper, B. Redundancy and divergence in the amyloid precursor protein family. FEBS Lett. 587, 2036–2045 (2013).
Muller, U. C., Deller, T. & Korte, M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 18, 281–298 (2017).
Puig, K. L. & Combs, C. K. Expression and function of APP and its metabolites outside the central nervous system. Exp. Gerontol. 48, 608–611 (2013).
Wang, A., Das, P., Switzer, R. C. 3rd, Golde, T. E. & Jankowsky, J. L. Robust amyloid clearance in a mouse model of Alzheimer's disease provides novel insights into the mechanism of amyloid-β immunotherapy. J. Neurosci. 31, 4124–4136 (2011).
Kogel, D., Deller, T. & Behl, C. Roles of amyloid precursor protein family members in neuroprotection, stress signaling and aging. Exp. Brain Res. 217, 471–479 (2012).
Klevanski, M. et al. Differential role of APP and APLPs for neuromuscular synaptic morphology and function. Mol. Cell. Neurosci. 61, 201–210 (2014).
Ludewig, S. & Korte, M. Novel insights into the physiological function of the APP (gene) family and its proteolytic fragments in synaptic plasticity. Front. Mol. Neurosci. 9, 161 (2016).
Hefter, D. et al. Amyloid precursor protein protects neuronal network function after hypoxia via control of voltage-gated calcium channels. J. Neurosci. 36, 8356–8371 (2016).
Grimm, M. O. et al. Intracellular APP domain regulates serine-palmitoyl-CoA transferase expression and is affected in Alzheimer's disease. Int. J. Alzheimers Dis. 2011, 695413 (2011).
Li, X. et al. Neuronal activity and secreted amyloid β lead to altered amyloid β precursor protein and presenilin 1 interactions. Neurobiol. Dis. 50, 127–134 (2013).
Kim, T. et al. Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science 341, 1399–1404 (2013).
Umeda, T., Mori, H., Zheng, H. & Tomiyama, T. Regulation of cholesterol efflux by amyloid β secretion. J. Neurosci. Res. 88, 1985–1994 (2010).
Chew, Y. L., Fan, X., Götz, J. & Nicholas, H. R. Protein with tau-like repeats regulates neuronal integrity and lifespan in C. elegans. J. Cell Sci. 126, 2079–2091 (2013).
Morris, M. et al. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 18, 1183–1189 (2015).
Wang, Y. & Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016).
Pooler, A. M., Phillips, E. C., Lau, D. H., Noble, W. & Hanger, D. P. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 14, 389–394 (2013).
Yamada, K. et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 211, 387–393 (2014).
Fa, M. et al. Extracellular tau oligomers produce an immediate impairment of LTP and memory. Sci. Rep. 6, 19393 (2016).
Violet, M. et al. A major role for tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front. Cell. Neurosci. 8, 84 (2014).
Ma, Q. L. et al. Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris water maze with aging. J. Neurosci. 34, 7124–7136 (2014).
DeVos, S. L. et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl Med. 9, eaag0481 (2017).
Liu, C., Song, X., Nisbet, R. & Götz, J. Co-immunoprecipitation with tau isoform-specific antibodies reveals distinct protein interactions, and highlights a putative role for 2N tau in disease. J. Biol. Chem. 291, 161–174 (2016).
Del Turco, D. et al. Region-specific differences in amyloid precursor protein expression in the mouse hippocampus. Front. Mol. Neurosci. 9, 134 (2016).
Szodorai, A. et al. APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle. J. Neurosci. 29, 14534–14544 (2009).
Wilhelm, B. G. et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344, 1023–1028 (2014).
Hoey, S. E., Williams, R. J. & Perkinton, M. S. Synaptic NMDA receptor activation stimulates α-secretase amyloid precursor protein processing and inhibits amyloid-β production. J. Neurosci. 29, 4442–4460 (2009).
Prox, J. et al. Postnatal disruption of the disintegrin/metalloproteinase ADAM10 in brain causes epileptic seizures, learning deficits, altered spine morphology, and defective synaptic functions. J. Neurosci. 33, 12915–12928 (2013).
Corbett, G. T., Gonzalez, F. J. & Pahan, K. Activation of peroxisome proliferator-activated receptor α stimulates ADAM10-mediated proteolysis of APP. Proc. Natl Acad. Sci. USA 112, 8445–8450 (2015).
Takagi-Niidome, S. et al. Cooperative roles of hydrophilic loop 1 and the C-terminus of presenilin 1 in the substrate-gating mechanism of γ-secretase. J. Neurosci. 35, 2646–2656 (2015).
Vassar, R. et al. Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J. Neurochem. 130, 4–28 (2014).
Takami, M. et al. γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. J. Neurosci. 29, 13042–13052 (2009).
Portelius, E. et al. A novel pathway for amyloid precursor protein processing. Neurobiol. Aging 32, 1090–1098 (2011).
Das, U. et al. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron 79, 447–460 (2013).
Yan, R. & Vassar, R. Targeting the β secretase BACE1 for Alzheimer's disease therapy. Lancet Neurol. 13, 319–329 (2014).
Sannerud, R. et al. Restricted location of PSEN2/γ-secretase determines substrate specificity and generates an intracellular Aβ pool. Cell 166, 193–208 (2016).
Kanatsu, K. et al. Decreased CALM expression reduces Aβ42 to total Aβ ratio through clathrin-mediated endocytosis of γ-secretase. Nat. Commun. 5, 3386 (2014).
Area-Gomez, E. et al. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am. J. Pathol. 175, 1810–1816 (2009).
Area-Gomez, E. et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 31, 4106–4123 (2012).
DeBoer, S. R., Dolios, G., Wang, R. & Sisodia, S. S. Differential release of β-amyloid from dendrite-versus axon-targeted APP. J. Neurosci. 34, 12313–12327 (2014).
Niederst, E. D., Reyna, S. M. & Goldstein, L. S. Axonal amyloid precursor protein and its fragments undergo somatodendritic endocytosis and processing. Mol. Biol. Cell 26, 205–217 (2015).
Nhan, H. S., Chiang, K. & Koo, E. H. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol. 129, 1–19 (2015).
Andrew, R. J., Kellett, K. A., Thinakaran, G. & Hooper, N. M. A. Greek tragedy: the growing complexity of alzheimer amyloid precursor protein proteolysis. J. Biol. Chem. 291, 19235–19244 (2016).
Kummer, M. P. & Heneka, M. T. Truncated and modified amyloid-β species. Alzheimers Res. Ther. 6, 28 (2014).
Barnham, K. J. et al. Neurotoxic, redox-competent Alzheimer's β-amyloid is released from lipid membrane by methionine oxidation. J. Biol. Chem. 278, 42959–42965 (2003).
Hou, L., Kang, I., Marchant, R. E. & Zagorski, M. G. Methionine 35 oxidation reduces fibril assembly of the amyloid Aβ1–42 peptide of Alzheimer's disease. J. Biol. Chem. 277, 40173–40176 (2002).
Wirths, O. et al. Intraneuronal pyroglutamate-Aβ3–42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 118, 487–496 (2009).
Alexandru, A. et al. Selective hippocampal neurodegeneration in transgenic mice expressing small amounts of truncated Aβ is induced by pyroglutamate-Aβ formation. J. Neurosci. 31, 12790–12801 (2011).
He, W. & Barrow, C. J. The Aβ 3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have greater β-sheet forming and aggregation propensities in vitro than full-length Aβ. Biochemistry 38, 10871–10877 (1999).
Nussbaum, J. M. et al. Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-β. Nature 485, 651–655 (2012).
Schilling, S. et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer's disease-like pathology. Nat. Med. 14, 1106–1111 (2008).
Kumar, S. et al. Extracellular phosphorylation of the amyloid β-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer's disease. EMBO J. 30, 2255–2265 (2011).
Kumar, S. et al. Phosphorylation of amyloid-β peptide at serine 8 attenuates its clearance via insulin-degrading and angiotensin-converting enzymes. J. Biol. Chem. 287, 8641–8651 (2012).
Kummer, M. P. et al. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron 71, 833–844 (2011).
Halim, A. et al. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid β-peptides in human cerebrospinal fluid. Proc. Natl Acad. Sci. USA 108, 11848–11853 (2011).
Chromy, B. A. et al. Self-assembly of Aβ1–42 into globular neurotoxins. Biochemistry 42, 12749–12760 (2003).
Lesne, S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006).
Shankar, G. M. et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 (2008).
Liu, P. et al. Quaternary structure defines a large class of amyloid-β oligomers neutralized by sequestration. Cell Rep. 11, 1760–1771 (2015).
Kayed, R. et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2, 18 (2007).
Friedrich, R. P. et al. Mechanism of amyloid plaque formation suggests an intracellular basis of Aβ pathogenicity. Proc. Natl Acad. Sci. USA 107, 1942–1947 (2010).
Dickson, D. W. et al. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol. Aging 16, 285–298 (1995).
Beach, T. G. et al. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer's disease: implications for amyloid imaging. J. Alzheimers Dis. 28, 869–876 (2012).
Nelson, P. T. et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381 (2012).
Bezprozvanny, I. Amyloid goes global. Sci. Signal. 2, e16 (2009).
Catalano, S. M. et al. The role of amyloid-β derived diffusible ligands (ADDLs) in Alzheimer's disease. Curr. Top. Med. Chem. 6, 597–608 (2006).
Lesne, S. E. et al. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain 136, 1383–1398 (2013).
Haass, C. & Selkoe, D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 (2007).
Murray, M. M. et al. Amyloid β protein: Aβ40 inhibits Aβ42 oligomerization. J. Am. Chem. Soc. 131, 6316–6317 (2009).
Al-Hilaly, Y. K. et al. A central role for dityrosine crosslinking of amyloid-β in Alzheimer's disease. Acta Neuropathol. Commun. 1, 83 (2013).
Thiabaud, G. et al. Heme binding induces dimerization and nitration of truncated β-amyloid peptide Aβ16 under oxidative stress. Angew. Chem. Int. Ed. Engl. 52, 8041–8044 (2013).
Shimizu, T., Fukuda, H., Murayama, S., Izumiyama, N. & Shirasawa, T. Isoaspartate formation at position 23 of amyloid β peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer's disease. J. Neurosci. Res. 70, 451–461 (2002).
Fonseca, M. I., Head, E., Velazquez, P., Cotman, C. W. & Tenner, A. J. The presence of isoaspartic acid in β-amyloid plaques indicates plaque age. Exp. Neurol. 157, 277–288 (1999).
Jawhar, S. et al. Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate Aβ formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J. Biol. Chem. 286, 4454–4460 (2011).
Solforosi, L., Milani, M., Mancini, N., Clementi, M. & Burioni, R. A closer look at prion strains: characterization and important implications. Prion 7, 99–108 (2013).
Petkova, A. T. et al. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science 307, 262–265 (2005).
Lu, J. X. et al. Molecular structure of β-amyloid fibrils in Alzheimer's disease brain tissue. Cell 154, 1257–1268 (2013).
Qiang, W., Yau, W. M., Lu, J. X., Collinge, J. & Tycko, R. Structural variation in amyloid-β fibrils from Alzheimer's disease clinical subtypes. Nature 541, 217–221 (2017).
Cohen, M. L. et al. Rapidly progressive Alzheimer's disease features distinct structures of amyloid-β. Brain 138, 1009–1022 (2015).
Selkoe, D. J. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav. Brain Res. 192, 106–113 (2008).
Walsh, D. M. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Cerpa, W., Dinamarca, M. C. & Inestrosa, N. C. Structure-function implications in Alzheimer's disease: effect of Aβ oligomers at central synapses. Curr. Alzheimer Res. 5, 233–243 (2008).
Tu, S., Okamoto, S., Lipton, S. A. & Xu, H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol. Neurodegener. 9, 48 (2014).
Lee, L. et al. Regulation of synaptic plasticity and cognition by SUMO in normal physiology and Alzheimer's disease. Sci. Rep. 4, 7190 (2014).
Reddy, P. H. Mitochondrial dysfunction in aging and Alzheimer's disease: strategies to protect neurons. Antioxid. Redox Signal. 9, 1647–1658 (2007).
Grimm, A., Friedland, K. & Eckert, A. Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer's disease. Biogerontology 17, 281–296 (2016).
Rhein, V. et al. Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc. Natl Acad. Sci. USA 106, 20057–20062 (2009).
Eckert, A. et al. Oligomeric and fibrillar species of β-amyloid (Aβ42) both impair mitochondrial function in P301L tau transgenic mice. J. Mol. Med. 86, 1255–1267 (2008).
Orrenius, S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 39, 443–455 (2007).
Smith, D. G., Cappai, R. & Barnham, K. J. The redox chemistry of the Alzheimer's disease amyloid β peptide. Biochim. Biophys. Acta 1768, 1976–1990 (2007).
Serra-Batiste, M. et al. Aβ42 assembles into specific β-barrel pore-forming oligomers in membrane-mimicking environments. Proc. Natl Acad. Sci. USA 113, 10866–10871 (2016).
Esposito, C. et al. Exploring interaction of β-amyloid segment (25–35) with membrane models through paramagnetic probes. J. Pept. Sci. 12, 766–774 (2006).
Lau, T. L. et al. Membrane interactions and the effect of metal ions of the amyloidogenic fragment Aβ25–35 in comparison to Aβ1–42 . Biochim. Biophys. Acta 1768, 2400–2408 (2007).
Arispe, N., Diaz, J. C. & Simakova, O. Aβ ion channels. Prospects for treating Alzheimer's disease with Aβ channel blockers. Biochim. Biophys. Acta 1768, 1952–1965 (2007).
Heneka, M. T. et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 14, 388–405 (2015).
Sondag, C. M., Dhawan, G. & Combs, C. K. β amyloid oligomers and fibrils stimulate differential activation of primary microglia. J. Neuroinflamm. 6, 1 (2009).
Pan, X. D. et al. Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer's disease. Mol. Neurodegener. 6, 45 (2011).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Buee, L. & Delacourte, A. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease. Brain Pathol. 9, 681–693 (1999).
Lacovich, V. et al. Tau isoforms imbalance impairs the axonal transport of the amyloid precursor protein in human neurons. J. Neurosci. 37, 58–69 (2017).
Ishigaki, S. et al. Altered tau isoform ratio caused by loss of FUS and SFPQ function leads to FTLD-like phenotypes. Cell Rep. 18, 1118–1131 (2017).
Ittner, L. M. et al. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc. Natl Acad. Sci. USA 105, 15997–16002 (2008).
Li, C. & Götz, J. Somatodendritic accumulation of tau in Alzheimer's disease is promoted by Fyn-mediated local protein translation. EMBO J. 36, 3120–3138 (2017).
Kopke, E. et al. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 268, 24374–24384 (1993).
Li, B., Chohan, M. O., Grundke-Iqbal, I. & Iqbal, K. Disruption of microtubule network by Alzheimer abnormally hyperphosphorylated tau. Acta Neuropathol. 113, 501–511 (2007).
Hoover, B. R. et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 (2010).
Xia, D., Li, C. & Götz, J. Pseudophosphorylation of tau at distinct epitopes or the presence of the P301L mutation targets the microtubule-associated protein tau to dendritic spines. Biochim. Biophys. Acta 1852, 913–924 (2015).
Lee, G., Newman, S. T., Gard, D. L., Band, H. & Panchamoorthy, G. Tau interacts with src-family non-receptor tyrosine kinases. J. Cell. Sci. 111, 3167–3177 (1998).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 (2010).
Usardi, A. et al. Tyrosine phosphorylation of tau regulates its interactions with Fyn SH2 domains, but not SH3 domains, altering the cellular localization of tau. FEBS J. 278, 2927–2937 (2011).
Novak, M., Jakes, R., Edwards, P. C., Milstein, C. & Wischik, C. M. Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc. Natl Acad. Sci. USA 88, 5837–5841 (1991).
Wei, Y. et al. Ribosylation triggering Alzheimer's disease-like tau hyperphosphorylation via activation of CaMKII. Aging Cell 14, 754–763 (2015).
Reyes, J. F., Fu, Y., Vana, L., Kanaan, N. M. & Binder, L. I. Tyrosine nitration within the proline-rich region of tau in Alzheimer's disease. Am. J. Pathol. 178, 2275–2285 (2011).
Zhang, Z. et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer's disease. Nat. Med. 20, 1254–1262 (2014).
Thomas, S. N. et al. Dual modification of Alzheimer's disease PHF-tau protein by lysine methylation and ubiquitylation: a mass spectrometry approach. Acta Neuropathol. 123, 105–117 (2012).
von Bergen, M., Barghorn, S., Biernat, J., Mandelkow, E. M. & Mandelkow, E. Tau aggregation is driven by a transition from random coil to β sheet structure. Biochim. Biophys. Acta 1739, 158–166 (2005).
Ganguly, P. et al. Tau assembly: the dominant role of PHF6 (VQIVYK) in microtubule binding region repeat R3. J. Phys. Chem. B 119, 4582–4593 (2015).
Stöhr, J. et al. A 31-residue peptide induces aggregation of tau's microtubule-binding region in cells. Nat. Chem. 9, 874–881 (2017).
Fagan, T. Monomeric seeds and oligomeric clouds — proteopathy news from AAIC (Alzheimer's Association International Conference) 2017. Alzforum: Networking for a cure http://www.alzforum.org/news/conference-coverage/monomeric-seeds-and-oligomeric-clouds-proteopathy-news-aaic (2017).
Soeda, Y. et al. Toxic tau oligomer formation blocked by capping of cysteine residues with 1,2-dihydroxybenzene groups. Nat. Commun. 6, 10216 (2015).
Usenovic, M. et al. Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J. Neurosci. 35, 14234–14250 (2015).
Lasagna-Reeves, C. A. et al. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 6, 39 (2011).
Castillo-Carranza, D. L. et al. Passive immunization with tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J. Neurosci. 34, 4260–4272 (2014).
Merino-Serrais, P. et al. The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer's disease. Brain 136, 1913–1928 (2013).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Nakamura, K. et al. Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer's disease. Cell 149, 232–244 (2012).
Kondo, A. et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436 (2015).
Bodea, L. G. et al. Accelerated aging exacerbates a pre-existing pathology in a tau transgenic mouse model. Aging Cell 16, 377–386 (2017).
Braak, H. & Braak, E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278 (1995).
Holmes, B. B. & Diamond, M. I. Prion-like properties of tau protein: the importance of extracellular tau as a therapeutic target. J. Biol. Chem. 289, 19855–19861 (2014).
Calafate, S. et al. Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep. 11, 1176–1183 (2015).
Mirbaha, H., Holmes, B. B., Sanders, D. W., Bieschke, J. & Diamond, M. I. Tau trimers are the minimal propagation unit spontaneously internalized to seed intracellular aggregation. J. Biol. Chem. 290, 14893–14903 (2015).
Lasagna-Reeves, C. A. et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2, 700 (2012).
Wu, J. W. et al. Small misfolded tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 288, 1856–1870 (2013).
Jackson, S. J. et al. Short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S tau. J. Neurosci. 36, 762–772 (2016).
Takeda, S. et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer's disease brain. Nat. Commun. 6, 8490 (2015).
Polanco, J. C., Scicluna, B. J., Hill, A. F. & Götz, J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem. 291, 12445–12466 (2016).
Wang, Y. et al. The release and trans-synaptic transmission of tau via exosomes. Mol. Neurodegener. 12, 5 (2017).
Pickett, E. K. et al. Spread of tau down neural circuits precedes synapse and neuronal loss in the rTgTauEC mouse model of early Alzheimer's disease. Synapse 71, e21965 (2017).
Hu, W. et al. Hyperphosphorylation determines both the spread and the morphology of tau pathology. Alzheimers Dement. 12, 1066–1077 (2016).
Sanders, D. W. et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014).
Kaufman, S. K. et al. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 92, 796–812 (2016).
Clavaguera, F. et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl Acad. Sci. USA 110, 9535–9540 (2013).
Polydoro, M. et al. Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer's disease model. Acta Neuropathol. 127, 257–270 (2014).
Zhou, L. et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 8, 15295 (2017).
Hatch, R. J., Wei, Y., Xia, D. & Götz, J. Hyperphosphorylated tau causes reduced hippocampal CA1 excitability by relocating the axon initial segment. Acta Neuropathol. 133, 717–730 (2017).
Regan, P. et al. Tau phosphorylation at serine 396 residue is required for hippocampal LTD. J. Neurosci. 35, 4804–4812 (2015).
Ittner, A. et al. Site-specific phosphorylation of tau inhibits amyloid-β toxicity in Alzheimer's mice. Science 354, 904–908 (2016).
Min, S. W. et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966 (2010).
Cohen, T. J. et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2, 252 (2011).
Irwin, D. J. et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain 135, 807–818 (2012).
Min, S. W. et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21, 1154–1162 (2015).
Tracy, T. E. et al. Acetylated tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes tauopathy-related memory loss. Neuron 90, 245–260 (2016).
Zhao, X. et al. Caspase-2 cleavage of tau reversibly impairs memory. Nat. Med. 22, 1268–1276 (2016).
Quintanilla, R. A., Matthews-Roberson, T. A., Dolan, P. J. & Johnson, G. V. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J. Biol. Chem. 284, 18754–18766 (2009).
Amadoro, G. et al. AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol. Dis. 62, 489–507 (2014).
Frost, B., Hemberg, M., Lewis, J. & Feany, M. B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 17, 357–366 (2014).
Mansuroglu, Z. et al. Loss of tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci. Rep. 6, 33047 (2016).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Maphis, N. et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138, 1738–1755 (2015).
Sanchez-Mejias, E. et al. Soluble phospho-tau from Alzheimer's disease hippocampus drives microglial degeneration. Acta Neuropathol. 132, 897–916 (2016).
Frandemiche, M. L. et al. Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-β oligomers. J. Neurosci. 34, 6084–6097 (2014).
Li, X. et al. Novel diffusion barrier for axonal retention of tau in neurons and its failure in neurodegeneration. EMBO J. 30, 4825–4837 (2011).
Sohn, P. D. et al. Acetylated tau destabilizes the cytoskeleton in the axon initial segment and is mislocalized to the somatodendritic compartment. Mol. Neurodegener. 11, 47 (2016).
Cochran, J. N., Hall, A. M. & Roberson, E. D. The dendritic hypothesis for Alzheimer's disease pathophysiology. Brain Res. Bull. 103, 18–28 (2014).
Mairet-Coello, G. et al. The CAMKK2–AMPK kinase pathway mediates the synaptotoxic effects of Aβ oligomers through tau phosphorylation. Neuron 78, 94–108 (2013).
Amar, F. et al. The amyloid-β oligomer Aβ*56 induces specific alterations in neuronal signaling that lead to tau phosphorylation and aggregation. Sci. Signal. 10, aal2021 (2017).
Shipton, O. A. et al. Tau protein is required for amyloid β-induced impairment of hippocampal long-term potentiation. J. Neurosci. 31, 1688–1692 (2011).
Zempel, H. et al. Amyloid-β oligomers induce synaptic damage via tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 32, 2920–2937 (2013).
Perez, M. J., Vergara-Pulgar, K., Jara, C., Cabezas-Opazo, F. & Quintanilla, R. A. Caspase-cleaved tau impairs mitochondrial dynamics in Alzheimer's disease. Mol. Neurobiol. http://dx.doi.org/10.1007/s12035-017-0385-x (2017).
Manczak, M. & Reddy, P. H. Abnormal interaction of VDAC1 with amyloid β and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease. Hum. Mol. Genet. 21, 5131–5146 (2012).
Vossel, K. A. et al. Tau reduction prevents Aβ-induced axonal transport deficits by blocking activation of GSK3β. J. Cell Biol. 209, 419–433 (2015).
Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 (2014).
Roberts, B. R. et al. Biochemically-defined pools of amyloid-β in sporadic Alzheimer's disease: correlation with amyloid PET. Brain 140, 1486–1498 (2017).
Bodea, L. G., Eckert, A., Ittner, L. M., Piguet, O. & Götz, J. Tau physiology and pathomechanisms in frontotemporal lobar degeneration. J. Neurochem. 138 (Suppl. 1), 71–94 (2016).
Aho, L., Pikkarainen, M., Hiltunen, M., Leinonen, V. & Alafuzoff, I. Immunohistochemical visualization of amyloid-β protein precursor and amyloid-β in extra- and intracellular compartments in the human brain. J. Alzheimers Dis. 20, 1015–1028 (2010).
Demattos, R. B. et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer's disease mice. Neuron 76, 908–920 (2012).
Götz, J. et al. Animal models reveal role for tau phosphorylation in human disease. Biochim. Biophys. Acta 1802, 860–871 (2010).
Luk, C. et al. Development and assessment of sensitive immuno-PCR assays for the quantification of cerebrospinal fluid three- and four-repeat tau isoforms in tauopathies. J. Neurochem. 123, 396–405 (2012).
Liu, C. & Götz, J. Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PLoS ONE 8, e84849 (2013).
Wolk, D. A. et al. Amyloid imaging in Alzheimer's disease: comparison of florbetapir and Pittsburgh compound-B positron emission tomography. J. Neurol. Neurosurg. Psychiatry 83, 923–926 (2012).
Clark, C. M. et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678 (2012).
Pontecorvo, M. J. et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 140, 748–763 (2017).
Khatoon, S., Chalbot, S., Bolognin, S., Puolivali, J. & Iqbal, K. Elevated tau level in aged rat cerebrospinal fluid reduced by treatment with a neurotrophic compound. J. Alzheimers Dis. 47, 557–564 (2015).
Lleo, A. et al. Cerebrospinal fluid biomarkers in trials for Alzheimer and Parkinson diseases. Nat. Rev. Neurol. 11, 41–55 (2015).
Huynh, R. A. & Mohan, C. Alzheimer's disease: biomarkers in the genome, blood, and cerebrospinal fluid. Front. Neurol. 8, 102 (2017).
Ewers, M. et al. CSF biomarkers for the differential diagnosis of Alzheimer's disease: a large-scale international multicenter study. Alzheimers Dement. 11, 1306–1315 (2015).
Vos, S. J. et al. Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology 80, 1124–1132 (2013).
Chouraki, V. et al. Plasma amyloid-β and risk of Alzheimer's disease in the Framingham Heart Study. Alzheimers Dement. 11, 249–257.e1 (2015).
Rembach, A. et al. Changes in plasma amyloid β in a longitudinal study of aging and Alzheimer's disease. Alzheimers Dement. 10, 53–61 (2014).
Olsson, B. et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 15, 673–684 (2016).
Kim, C. B., Choi, Y. Y., Song, W. K. & Song, K. B. Antibody-based magnetic nanoparticle immunoassay for quantification of Alzheimer's disease pathogenic factor. J. Biomed. Opt. 19, 051205 (2014).
Shi, M. et al. Salivary tau species are potential biomarkers of Alzheimer's disease. J. Alzheimers Dis. 27, 299–305 (2011).
Carro, E. et al. Early diagnosis of mild cognitive impairment and Alzheimer's disease based on salivary lactoferrin. Alzheimers Dement. 8, 131–138 (2017).
Hampel, H. et al. Precision medicine — the golden gate for detection, treatment and prevention of Alzheimer's disease. J. Prev. Alzheimers Dis. 3, 243–259 (2016).
Takata, M. et al. Detection of amyloid β protein in the urine of Alzheimer's disease patients and healthy individuals. Neurosci. Lett. 435, 126–130 (2008).
Zhou, Y. et al. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci. Rep. 6, 35186 (2016).
Wang, S. X. et al. Detection of the tau protein in human serum by a sensitive four-electrode electrochemical biosensor. Biosens. Bioelectron. 92, 482–488 (2017).
Tiiman, A., Jarvet, J., Graslund, A. & Vukojevic, V. Heterogeneity and turnover of intermediates during amyloid-β (Aβ) peptide aggregation studied by fluorescence correlation spectroscopy. Biochemistry 54, 7203–7211 (2015).
Ly, S. et al. Binding of apolipoprotein E inhibits the oligomer growth of amyloid-β peptide in solution as determined by fluorescence cross-correlation spectroscopy. J. Biol. Chem. 288, 11628–11635 (2013).
Pujol-Pina, R. et al. SDS-PAGE analysis of Aβ oligomers is disserving research into Alzheimer's disease: appealing for ESI-IM-MS. Sci. Rep. 5, 14809 (2015).
Mair, W. et al. FLEXItau: quantifying post-translational modifications of tau protein in vitro and in human disease. Anal. Chem 88, 3704–3714 (2016).
Buividas, R. et al. Statistically quantified measurement of an Alzheimer's marker by surface-enhanced Raman scattering. J. Biophoton. 8, 567–574 (2015).
Schedin-Weiss, S., Caesar, I., Winblad, B., Blom, H. & Tjernberg, L. O. Super-resolution microscopy reveals γ-secretase at both sides of the neuronal synapse. Acta Neuropathol. Commun. 4, 29 (2016).
Zhang, W. I. et al. Super-resolution microscopy of cerebrospinal fluid biomarkers as a tool for Alzheimer's disease diagnostics. J. Alzheimers Dis. 46, 1007–1020 (2015).
Bolmont, T. et al. Label-free imaging of cerebral β-amyloidosis with extended-focus optical coherence microscopy. J. Neurosci. 32, 14548–14556 (2012).
Tam, J. H., Seah, C. & Pasternak, S. H. The amyloid precursor protein is rapidly transported from the Golgi apparatus to the lysosome where it is processed into β-amyloid. Mol. Brain 7, 54 (2014).
Xia, D., Gutmann, J. M. & Götz, J. Mobility and subcellular localization of endogenous, gene-edited tau differs from that of over-expressed human wild-type and P301L mutant tau. Sci. Rep. 6, 29074 (2016).
Godin, A. G. et al. Spatial intensity distribution analysis reveals abnormal oligomerization of proteins in single cells. Biophys. J. 109, 710–721 (2015).
Dorosh, L. & Stepanova, M. Probing oligomerization of amyloid β peptide in silico. Mol. Biosyst. 13, 165–182 (2016).
Proctor, C. J., Boche, D., Gray, D. A. & Nicoll, J. A. Investigating interventions in Alzheimer's disease with computer simulation models. PLoS ONE 8, e73631 (2013).
Fitzpatrick, A. W. P. et al. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature 547, 185–190 (2017).
Abbott, A. & Dolgin, E. Failed Alzheimer's trial does not kill leading theory of disease. Nature 540, 15–16 (2016).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 537, 50–56 (2016).
Jonsson, T. et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature 488, 96–99 (2012).
McDade, E. & Bateman, R. J. Stop Alzheimer's before it starts. Nature 547, 153–155 (2017).
Kanodia, J. S. et al. Prospective design of anti-transferrin receptor bispecific antibodies for optimal delivery into the human brain. CPT Pharmacometr. Syst. Pharmacol. 5, 283–291 (2016).
Lee, C. C. et al. Design and optimization of anti-amyloid domain antibodies specific for β-amyloid and islet amyloid polypeptide. J. Biol. Chem. 291, 2858–2873 (2016).
Debie, P. et al. Effect of dye and conjugation chemistry on the biodistribution profile of near-infrared-labeled nanobodies as tracers for image-guided surgery. Mol. Pharm. 14, 1145–1153 (2017).
Leinenga, G., Langton, C., Nisbet, R. & Götz, J. Ultrasound treatment of neurological diseases — current and emerging applications. Nat. Rev. Neurol. 12, 161–174 (2016).
Jordao, J. F. et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 248, 16–29 (2013).
Leinenga, G. & Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model. Sci. Transl. Med. 7, 278ra33 (2015).
Nisbet, R. M. et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 140, 1220–1230 (2017).
Meredith, S. C. Protein denaturation and aggregation: cellular responses to denatured and aggregated proteins. Ann. NY Acad. Sci. 1066, 181–221 (2005).
Scheper, W., Nijholt, D. A. & Hoozemans, J. J. The unfolded protein response and proteostasis in Alzheimer disease: preferential activation of autophagy by endoplasmic reticulum stress. Autophagy 7, 910–911 (2011).
Martin, S. et al. Increased polyubiquitination and proteasomal degradation of a Munc18-1 disease-linked mutant causes temperature-sensitive defect in exocytosis. Cell Rep. 9, 206–218 (2014).
Forloni, G., Artuso, V., La Vitola, P. & Balducci, C. Oligomeropathies and pathogenesis of Alzheimer and Parkinson's diseases. Mov. Disord. 31, 771–781 (2016).
Oikawa, T. et al. α-Synuclein fibrils exhibit gain of toxic function, promoting tau aggregation and inhibiting microtubule assembly. J. Biol. Chem. 291, 15046–15056 (2016).
Baker, S. & Götz, J. What we can learn from animal models about cerebral multi-morbidity. Alzheimers Res. Ther. 7, 11 (2015).
Chai, Y. J. et al. Munc18-1 is a molecular chaperone for α-synuclein, controlling its self-replicating aggregation. J. Cell Biol. 214, 705–718 (2016).
Harper, J. D., Wong, S. S., Lieber, C. M. & Lansbury, P. T. Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem. Biol. 4, 119–125 (1997).
Rosen, R. F. et al. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J. Neurochem. 120, 660–666 (2012).
Nath, S. et al. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of β-amyloid. J. Neurosci. 32, 8767–8777 (2012).
Domert, J. et al. Spreading of amyloid-β peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol. Dis. 65, 82–92 (2014).
Clavaguera, F. et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 (2009).
Guo, J. L. et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154, 103–117 (2013).
Rutherford, N. J. et al. Comparison of the in vivo induction and transmission of α-synuclein pathology by mutant α-synuclein fibril seeds in transgenic mice. Hum. Mol. Genet. http://dx.doi.org/10.1093/hmg/ddx371 (2017).
Sano, K. et al. Prion-like seeding of misfolded α-synuclein in the brains of dementia with Lewy body patients in RT-QUIC. Mol. Neurobiol. http://dx.doi.org/10.1007/s12035-017-0624-1 (2017).
Aulic, S. et al. α-Synuclein amyloids hijack prion protein to gain cell entry, facilitate cell-to-cell spreading and block prion replication. Sci. Rep. 7, 10050 (2017).
Peled, S. et al. Single cell imaging and quantification of TDP-43 and α-synuclein intercellular propagation. Sci. Rep. 7, 544 (2017).
Braak, H. & Del Tredici, K. Neuropathological staging of brain pathology in sporadic Parkinson's disease: separating the wheat from the chaff. J. Parkinsons Dis. 7, S73–S87 (2017).
Munch, C., O'Brien, J. & Bertolotti, A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl Acad. Sci. USA 108, 3548–3553 (2011).
Furukawa, Y., Kaneko, K., Watanabe, S., Yamanaka, K. & Nukina, N. A seeding reaction recapitulates intracellular formation of sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J. Biol. Chem. 286, 18664–18672 (2011).
Smethurst, P. et al. In vitro prion-like behaviour of TDP-43 in ALS. Neurobiol. Dis. 96, 236–247 (2016).
Nonaka, T. et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 4, 124–134 (2013).
Brettschneider, J. et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 74, 20–38 (2013).
Feuillette, S. et al. Neuron-to-neuron transfer of FUS in Drosophila primary neuronal culture is enhanced by ALS-associated mutations. J. Mol. Neurosci. 62, 114–122 (2017).
Tan, Z. et al. Huntington's disease cerebrospinal fluid seeds aggregation of mutant huntingtin. Mol. Psychiatry 20, 1286–1293 (2015).
Serpionov, G. V., Alexandrov, A. I., Antonenko, Y. N. & Ter-Avanesyan, M. D. A protein polymerization cascade mediates toxicity of non-pathological human huntingtin in yeast. Sci. Rep. 5, 18407 (2015).
Ren, P. H. et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol. 11, 219–225 (2009).
Babcock, D. T. & Ganetzky, B. Transcellular spreading of huntingtin aggregates in the Drosophila brain. Proc. Natl Acad. Sci. USA 112, E5427–E5433 (2015).
Pecho-Vrieseling, E. et al. Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat. Neurosci. 17, 1064–1072 (2014).
Come, J. H., Fraser, P. E. & Lansbury, P. T. Jr. A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc. Natl Acad. Sci. USA 90, 5959–5963 (1993).
Peden, A. H. et al. Sensitive and specific detection of sporadic Creutzfeldt–Jakob disease brain prion protein using real-time quaking-induced conversion. J. Gen. Virol. 93, 438–449 (2012).
Silveira, J. R. et al. The most infectious prion protein particles. Nature 437, 257–261 (2005).
Buyukmihci, N., Goehring-Harmon, F. & Marsh, R. F. Neural pathogenesis of experimental scrapie after intraocular inoculation of hamsters. Exp. Neurol. 81, 396–406 (1983).
Fraser, H. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature 295, 149–150 (1982).
Acknowledgements
J.G. is supported by the Estate of Clem Jones AO, the Australian Research Council (grant DP160103812) and the National Health and Medical Research Council of Australia (NHMRC; grants GNT1037746 and GNT1127999). F.A.M. is supported by the Australian Research Council (grants DP170100125, LE0882864 and LE130100078) and the NHMRC (grant GNT1058769 and NHMRC Senior Research Fellowship GNT1060075). L.-G.B. is supported by the Peter Hilton Fellowship. The authors thank R. Tweedale for critically reading the manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, wrote the manuscript, made substantial contributions to discussions of its content and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Long-term potentiation
-
A cellular mechanism underlying learning and memory that involves a persistent increase in synaptic strength following high-frequency stimulation.
- Sumoylation
-
A post-translational modification involving conjugation with small ubiquitin-like modifiers (SUMOs).
Rights and permissions
About this article
Cite this article
Polanco, J., Li, C., Bodea, LG. et al. Amyloid-β and tau complexity — towards improved biomarkers and targeted therapies. Nat Rev Neurol 14, 22–39 (2018). https://doi.org/10.1038/nrneurol.2017.162
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.162
This article is cited by
-
Distinguishing IDH mutation status in gliomas using FTIR-ATR spectra of peripheral blood plasma indicating clear traces of protein amyloid aggregation
BMC Cancer (2024)
-
Scanning ultrasound-mediated memory and functional improvements do not require amyloid-β reduction
Molecular Psychiatry (2024)
-
Modulation of neural circuits by melatonin in neurodegenerative and neuropsychiatric disorders
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Tau forms synaptic nano-biomolecular condensates controlling the dynamic clustering of recycling synaptic vesicles
Nature Communications (2023)
-
Clinical relevance of animal models in aging-related dementia research
Nature Aging (2023)