Abstract
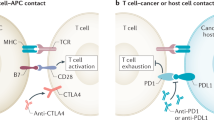
Cancer treatment strategies based on immune stimulation have recently entered the clinical arena, with unprecedented success. Immune checkpoint inhibitors (ICIs) work by indiscriminately promoting immune responses, which target tumour-associated antigens or tumour-specific mutations. However, the augmented immune response, most notably the T cell response, can cause either direct neurotoxicity or, more commonly, indirect neurotoxic effects through systemic or local inflammatory mechanisms or autoimmune mechanisms. Consequently, patients treated with ICIs are susceptible to CNS disease, including paraneoplastic neurological syndromes, encephalitis, multiple sclerosis and hypophysitis. In this Opinion article, we introduce the mechanisms of action of ICIs and review their adverse effects on the CNS. We highlight the importance of early detection of these neurotoxic effects, which should be distinguished from brain metastasis, and the need for early detection of neurotoxicity. It is crucial that physicians are well informed of these neurological adverse effects, given the anticipated increase in the use of immunotherapies to treat cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Allison, J. P. Checkpoints. Cell 162, 1202–1205 (2015).
Tran, E., Robbins, P. F. & Rosenberg, S. A. 'Final common pathway' of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 18, 255–262 (2017).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13, 227–242 (2013).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Schadendorf, D. et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33, 1889–1894 (2015).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Butte, M. J., Keir, M. E., Phamduy, T. B., Sharpe, A. H. & Freeman, G. J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122 (2007).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Anderson, A. C., Joller, N. & Kuchroo, V. K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016).
Nowak, E. C. et al. Immunoregulatory functions of VISTA. Immunol. Rev. 276, 66–79 (2017).
Cuzzubbo, S. et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur. J. Cancer 73, 1–8 (2017).
Wick, W., Hertenstein, A. & Platten, M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 17, e529–e541 (2016).
Hottinger, A. F. Neurologic complications of immune checkpoint inhibitors. Curr. Opin. Neurol. 29, 806–812 (2016).
Spain, L. et al. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann. Oncol. 28, 377–385 (2017).
Gerdes, L. A. et al. CTLA4 as immunological checkpoint in the development of multiple sclerosis. Ann. Neurol. 80, 294–300 (2016).
Larkin, J. et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist 22, 709–718 (2017).
Williams, T. J. et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 73, 928–933 (2016).
Gettings, E. J., Hackett, C. T. & Scott, T. F. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult. Scler. 21, 670 (2015).
Wilson, M. A. et al. Acute visual loss after ipilimumab treatment for metastatic melanoma. J. Immunother. Cancer 4, 66 (2016).
Wilgenhof, S. & Neyns, B. Anti-CTLA-4 antibody-induced Guillain–Barré syndrome in a melanoma patient. Ann. Oncol. 22, 991–993 (2011).
Liao, B., Shroff, S., Kamiya-Matsuoka, C. & Tummala, S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 16, 589–593 (2014).
Bompaire, F. et al. Severe meningo-radiculo-neuritis associated with ipilimumab. Invest. New Drugs 30, 2407–2410 (2012).
Thaipisuttikul, I., Chapman, P. & Avila, E. K. Peripheral neuropathy associated with ipilimumab: a report of 2 cases. J. Immunother. 38, 77–79 (2015).
Loochtan, A. I., Nickolich, M. S. & Hobson-Webb, L. D. Myasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancer. Muscle Nerve 52, 307–308 (2015).
Cao, Y. et al. CNS demyelination and enhanced myelin-reactive responses after ipilimumab treatment. Neurology 86, 1553–1556 (2016).
Maurice, C. et al. Subacute CNS demyelination after treatment with nivolumab for melanoma. Cancer Immunol. Res. 3, 1299–1302 (2015).
Brown, M. P., Hissaria, P., Hsieh, A. H., Kneebone, C. & Vallat, W. Autoimmune limbic encephalitis with anti-contactin-associated protein-like 2 antibody secondary to pembrolizumab therapy. J. Neuroimmunol. 305, 16–18 (2017).
Bossart, S. et al. Case report: encephalitis, with brainstem involvement, following checkpoint inhibitor therapy in metastatic melanoma. Oncologist 22, 749–753 (2017).
Yshii, L. M. et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain 139, 2923–2934 (2016).
Waisman, A., Liblau, R. S. & Becher, B. Innate and adaptive immune responses in the CNS. Lancet Neurol. 14, 945–955 (2015).
Salama, A. D. et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 198, 71–78 (2003).
Rui, Y., Honjo, T. & Chikuma, S. Programmed cell death 1 inhibits inflammatory helper T-cell development through controlling the innate immune response. Proc. Natl Acad. Sci. USA 110, 16073–16078 (2013).
Kroner, A. et al. Accelerated course of experimental autoimmune encephalomyelitis in PD-1-deficient central nervous system myelin mutants. Am. J. Pathol. 174, 2290–2299 (2009).
Jiang, T. T. et al. Programmed death-1 culls peripheral accumulation of high-affinity autoreactive CD4 T cells to protect against autoimmunity. Cell Rep. 17, 1783–1794 (2016).
Vincent, A., Lang, B. & Newsom-Davis, J. Autoimmunity to the voltage-gated calcium channel underlies the Lambert–Eaton myasthenic syndrome, a paraneoplastic disorder. Trends Neurosci. 12, 496–502 (1989).
Darnell, R. B. & Posner, J. B. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 349, 1543–1554 (2003).
Darnell, R. B. & Posner, J. B. Observing the invisible: successful tumor immunity in humans. Nat. Immunol. 4, 201 (2003).
Jaeckle, K. A. et al. Autoimmune response of patients with paraneoplastic cerebellar degeneration to a Purkinje cell cytoplasmic protein antigen. Ann. Neurol. 18, 592–600 (1985).
Dalmau, J., Furneaux, H. M., Gralla, R. J., Kris, M. G. & Posner, J. B. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer — a quantitative western blot analysis. Ann. Neurol. 27, 544–552 (1990).
Pignolet, B. S., Gebauer, C. M. & Liblau, R. S. Immunopathogenesis of paraneoplastic neurological syndromes associated with anti-Hu antibodies: a beneficial antitumor immune response going awry. Oncoimmunology 2, e27384 (2013).
Steinman, L. Conflicting consequences of immunity to cancer versus autoimmunity to neurons: insights from paraneoplastic disease. Eur. J. Immunol. 44, 3201–3205 (2014).
Schneider, S., Potthast, S., Komminoth, P., Schwegler, G. & Bohm, S. PD-1 checkpoint inhibitor associated autoimmune encephalitis. Case Rep. Oncol. 10, 473–478 (2017).
Laubli, H. et al. Cerebral vasculitis mimicking intracranial metastatic progression of lung cancer during PD-1 blockade. J. Immunother. Cancer 5, 46 (2017).
Salam, S., Lavin, T. & Turan, A. Limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep. http://dx.doi.org/10.1136/bcr-2016-215012 (2016).
Arriola, E. et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J. Thorac. Oncol. 11, 1511–1521 (2016).
Antonia, S. J. et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 17, 883–895 (2016).
Ben-Nun, A. et al. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J. Autoimmun. 54, 33–50 (2014).
Carter, L. L. et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 182, 124–134 (2007).
Takizawa, S. et al. Role of the programmed death-1 (PD-1) pathway in regulation of Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Neuroimmunol. 274, 78–85 (2014).
Hohlfeld, R., Dornmair, K., Meinl, E. & Wekerle, H. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 15, 198–209 (2016).
Hohlfeld, R., Dornmair, K., Meinl, E. & Wekerle, H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 15, 317–331 (2016).
Balar, A. V. & Weber, J. S. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol. Immunother. 66, 551–564 (2017).
Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Dillard, T., Yedinak, C. G., Alumkal, J. & Fleseriu, M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 13, 29–38 (2010).
Byun, D. J., Wolchok, J. D., Rosenberg, L. M. & Girotra, M. Cancer immunotherapy — immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 13, 195–207 (2017).
Albarel, F. et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur. J. Endocrinol. 172, 195–204 (2015).
Okano, Y. et al. Nivolumab-induced hypophysitis in a patient with advanced malignant melanoma. Endocr. J. 63, 905–912 (2016).
Caturegli, P. et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am. J. Pathol. 186, 3225–3235 (2016).
Iwama, S. et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6, 230ra45 (2014).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Bot, I., Blank, C. U., Boogerd, W. & Brandsma, D. Neurological immune-related adverse events of ipilimumab. Pract. Neurol. 13, 278–280 (2013).
Maur, M. et al. Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J. Clin. Oncol. 30, e76–e78 (2012).
Bien, C. G. et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135, 1622–1638 (2012).
Johnson, D. B. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2, 234–240 (2016).
Menzies, A. M. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 28, 368–376 (2017).
Eggermont, A. M. et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375, 1845–1855 (2016).
Dansokho, C. et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251 (2016).
Baruch, K. et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat. Med. 22, 135–137 (2016).
Goldberg, S. B. et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 17, 976–983 (2016).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Schildberg, F. A., Klein, S. R., Freeman, G. J. & Sharpe, A. H. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 44, 955–972 (2016).
Boussiotis, V. A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 375, 1767–1778 (2016).
Croft, M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 (2010).
Knee, D. A., Hewes, B. & Brogdon, J. L. Rationale for anti-GITR cancer immunotherapy. Eur. J. Cancer 67, 1–10 (2016).
Denoeud, J. & Moser, M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J. Leukoc. Biol. 89, 195–203 (2011).
Bartkowiak, T. & Curran, M. A. 4-1BB agonists: multi-potent potentiators of tumor immunity. Front. Oncol. 5, 117 (2015).
Sanchez-Paulete, A. R. et al. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur. J. Immunol. 46, 513–522 (2016).
Quezada, S. A., Jarvinen, L. Z., Lind, E. F. & Noelle, R. J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328 (2004).
Yuan, J. et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J. Immunother. Cancer 4, 3 (2016).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Pitt, J. M. et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44, 1255–1269 (2016).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017).
Kamphorst, A. O. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Mishra, V., Schuetz, H. & Haorah, J. Differential induction of PD-1/PD-L1 in neuroimmune cells by drug of abuse. Int. J. Physiol. Pathophysiol. Pharmacol. 7, 87–97 (2015).
Chen, L. et al. Constitutive neuronal expression of the immune regulator, programmed death 1 (PD-1), identified during experimental autoimmune uveitis. Ocul. Immunol. Inflamm. 17, 47–55 (2009).
Ren, X., Akiyoshi, K., Vandenbark, A. A., Hurn, P. D. & Offner, H. Programmed death-1 pathway limits central nervous system inflammation and neurologic deficits in murine experimental stroke. Stroke 42, 2578–2583 (2011).
Phares, T. W. et al. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. J. Immunol. 182, 5430–5438 (2009).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Schreiner, B., Bailey, S. L., Shin, T., Chen, L. & Miller, S. D. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 38, 2706–2717 (2008).
Pittet, C. L., Newcombe, J., Prat, A. & Arbour, N. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J. Neuroinflammation 8, 155 (2011).
O'Keeffe, G. W., Gutierrez, H., Pandolfi, P. P., Riccardi, C. & Davies, A. M. NGF-promoted axon growth and target innervation requires GITRL–GITR signaling. Nat. Neurosci. 11, 135–142 (2008).
Hwang, H., Lee, S., Lee, W. H., Lee, H. J. & Suk, K. Stimulation of glucocorticoid-induced tumor necrosis factor receptor family-related protein ligand (GITRL) induces inflammatory activation of microglia in culture. J. Neurosci. Res. 88, 2188–2196 (2010).
Anderson, A. C. et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318, 1141–1143 (2007).
Wei, D., Ren, C., Chen, X. & Zhao, H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS ONE 7, e30892 (2012).
Mao, X. et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, aah3374 (2016).
Abdel-Haq, N., Hao, H. N. & Lyman, W. D. Cytokine regulation of CD40 expression in fetal human astrocyte cultures. J. Neuroimmunol. 101, 7–14 (1999).
Tan, J. et al. CD40 is expressed and functional on neuronal cells. EMBO J. 21, 643–652 (2002).
Ponomarev, E. D., Shriver, L. P. & Dittel, B. N. CD40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J. Immunol. 176, 1402–1410 (2006).
Omari, K. M. & Dorovini-Zis, K. CD40 expressed by human brain endothelial cells regulates CD4+ T cell adhesion to endothelium. J. Neuroimmunol. 134, 166–178 (2003).
Reali, C. et al. Expression of CD137 and its ligand in human neurons, astrocytes, and microglia: modulation by FGF-2. J. Neurosci. Res. 74, 67–73 (2003).
Blank, A. E. et al. Tumour necrosis factor receptor superfamily member 9 (TNFRSF9) is up-regulated in reactive astrocytes in human gliomas. Neuropathol. Appl. Neurobiol. 41, e56–e67 (2015).
Acknowledgements
The authors' work is supported by INSERM, the French National Centre for Scientific Research (CNRS) and Toulouse III University. R.S.L. is supported by grants from Fondation pour l'Aide à la Recherche sur la Sclérose en Plaques (ARSEP), Midi-Pyrénées Region, Agence Nationale de la Recherche (ANR) T cell-Mig, FP7-PEOPLE-2012-ITN NeuroKine, European Research Area Network (ERA-NET) Meltra-BBB, L'Association pour la Recherche sur le Cancer (ARC) Cancer Research Foundation and Ligue Régionale Contre le Cancer. R.H. is supported by the German Research Foundation (DFG; SFB-TRR128; SyNergy, EXC 1010), German Ministry for Education and Research (BMBF; German Competence Network Multiple Sclerosis), Werner Reichenberger Stiftung, Cyliax Stiftung and Verein Therapieforschung für Multiple Sklerose Kranke. The funding sources had no role in the writing of the manuscript or the decision to submit it for publication. We thank C. Robert, A. Dejean, D. Dunia, G. Martin-Blondel and S. Valitutti for insightful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
L.M.Y., R.H. and R.S.L. wrote the article. All authors made substantial contributions to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Ongoing or completed immune checkpoint molecule clinical trials (DOC 83 kb)
Rights and permissions
About this article
Cite this article
Yshii, L., Hohlfeld, R. & Liblau, R. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol 13, 755–763 (2017). https://doi.org/10.1038/nrneurol.2017.144
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.144
This article is cited by
-
The immunopathogenesis of narcolepsy type 1
Nature Reviews Immunology (2024)
-
Expression analysis of inhibitory B7 family members in Alzheimer’s disease
Metabolic Brain Disease (2023)
-
False-reactive hepatitis B surface antigen test results in cancer patients
European Journal of Clinical Microbiology & Infectious Diseases (2023)
-
Neurology of cancer immunotherapy
Neurological Sciences (2023)
-
A Case of Anti-Ma2 Encephalitis Presenting with Pendular Torsional Nystagmus
The Cerebellum (2023)