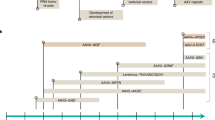
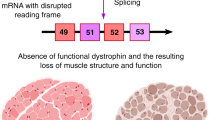
Key Points
-
Genetic neuromuscular conditions are debilitating and often prematurely fatal, and few standard treatment options are available
-
Genome engineering provides a viable method of correcting the underlying genetic mutations in neuromuscular diseases
-
A recent discovery, the highly adaptable CRISPR–Cas9 system, is already finding widespread use for cell therapy and in vivo gene editing
-
Ongoing challenges include efficient gene delivery, identification and reduction of off-target interactions, and immunogenicity of genome engineering tools, delivery vectors and other neoantigens
Abstract
For many neuromuscular disorders, including Duchenne muscular dystrophy, spinal muscular atrophy and myotonic dystrophy, the genetic causes are well known. Gene therapy holds promise for the treatment of these monogenic neuromuscular diseases, and many such therapies have made substantial strides toward clinical translation. Recently, genome engineering tools, including targeted gene editing and gene regulation, have become available to correct the underlying genetic mutations that cause these diseases. In particular, meganucleases, zinc finger nucleases, TALENs, and the CRISPR–Cas9 system have been harnessed to make targeted and specific modifications to the genome. However, for most gene therapy applications, including genome engineering, gene delivery remains the primary hurdle to clinical translation. In preclinical models, genome engineering tools have been delivered via gene-modified cells or by non-viral or viral vectors to correct a diverse array of genetic diseases. In light of the positive results of these studies, genome engineering therapies are being enthusiastically explored for several genetic neuromuscular disorders. This Review summarizes the genome engineering strategies that are currently under preclinical evaluation for the treatment of degenerative neuromuscular disorders, with a focus on the molecular tools that show the greatest potential for clinical translation of these therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stoddard, B. L. Homing endonucleases: from microbial genetic invaders to reagents for targeted DNA modification. Structure 19, 7–15 (2011).
Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S. & Gregory, P. D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 11, 636–646 (2010).
Gersbach, C. A., Gaj, T. & Barbas, C. F. 3rd. Synthetic zinc finger proteins: the advent of targeted gene regulation and genome modification technologies. Acc. Chem. Res. 47, 2309–2318 (2014).
Bogdanove, A. J. & Voytas, D. F. TAL effectors: customizable proteins for DNA targeting. Science 333, 1843–1846 (2011).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR–Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Cox, D. B., Platt, R. J. & Zhang, F. Therapeutic genome editing: prospects and challenges. Nat. Med. 21, 121–131 (2015).
Maeder, M. L. & Gersbach, C. A. Genome-editing technologies for gene and cell therapy. Mol. Ther. 24, 430–446 (2016).
Li, H. et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 475, 217–221 (2011).
Genovese, P. et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510, 235–240 (2014).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910 (2014).
Cirak, S. et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 378, 595–605 (2011).
Bowles, D. E. et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 20, 443–455 (2012).
van Deutekom, J. C. et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N. Engl. J. Med. 357, 2677–2686 (2007).
Goemans, N. M. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N. Engl. J. Med. 364, 1513–1522 (2011).
Finkel, R. S. et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dystrophy. PLoS ONE 8, e81302 (2013).
Tinsley, J. M. et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS ONE 6, e19189 (2011).
Amenta, A. R. et al. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc. Natl Acad. Sci. USA 108, 762–767 (2011).
Wood, M. J. Toward an oligonucleotide therapy for Duchenne muscular dystrophy: a complex development challenge. Sci. Transl Med. 2, 25ps15 (2010).
Lu, Q. L. et al. The status of exon skipping as a therapeutic approach to duchenne muscular dystrophy. Mol. Ther. 19, 9–15 (2011).
Verhaart, I. E. & Aartsma-Rus, A. Gene therapy for Duchenne muscular dystrophy. Curr. Opin. Neurol. 25, 588–596 (2012).
Foster, H., Popplewell, L. & Dickson, G. Genetic therapeutic approaches for Duchenne muscular dystrophy. Hum. Gene Ther. 23, 676–687 (2012).
Seto, J. T., Bengtsson, N. E. & Chamberlain, J. S. Therapy of genetic disorders — novel therapies for Duchenne muscular dystrophy. Curr. Pediatr. Rep. 2, 102–112 (2014).
Al-Zaidy, S., Rodino-Klapac, L. & Mendell, J. R. Gene therapy for muscular dystrophy: moving the field forward. Pediatr. Neurol. 51, 607–618 (2014).
Faravelli, I., Nizzardo, M., Comi, G. P. & Corti, S. Spinal muscular atrophy — recent therapeutic advances for an old challenge. Nat. Rev. Neurol. 11, 351–359 (2015).
Klein, A. F., Dastidar, S., Furling, D. & Chuah, M. K. Therapeutic approaches for dominant muscle diseases: highlight on myotonic dystrophy. Curr. Gene Ther. 15, 329–337 (2015).
Bonnemann, C. G. The collagen VI-related myopathies: muscle meets its matrix. Nat. Rev. Neurol. 7, 379–390 (2011).
Ross, C. A. et al. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 10, 204–216 (2014).
Smithies, O., Gregg, R. G., Boggs, S. S., Koralewski, M. A. & Kucherlapati, R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature 317, 230–234 (1985).
Thomas, K. R., Folger, K. R. & Capecchi, M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell 44, 419–428 (1986).
Rouet, P., Smih, F. & Jasin, M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl Acad. Sci. USA 91, 6064–6068 (1994).
Porteus, M. H. & Baltimore, D. Chimeric nucleases stimulate gene targeting in human cells. Science 300, 763 (2003).
Perez-Pinera, P., Ousterout, D. G., Brown, M. T. & Gersbach, C. A. Gene targeting to the ROSA26 locus directed by engineered zinc finger nucleases. Nucleic Acids Res. 40, 3741–3752 (2012).
Hermann, M. et al. Evaluation of OPEN zinc finger nucleases for direct gene targeting of the ROSA26 locus in mouse embryos. PLoS ONE 7, e41796 (2012).
Hockemeyer, D. et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 27, 851–857 (2009).
DeKelver, R. C. et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 20, 1133–1142 (2010).
Lee, H. J., Kim, E. & Kim, J. S. Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res. 20, 81–89 (2010).
Sollu, C. et al. Autonomous zinc-finger nuclease pairs for targeted chromosomal deletion. Nucleic Acids Res. 38, 8269–8276 (2010).
Colleaux, L. et al. Universal code equivalent of a yeast mitochondrial intron reading frame is expressed into E. coli as a specific double strand endonuclease. Cell 44, 521–533 (1986).
Silva, G. et al. Meganucleases and other tools for targeted genome engineering: perspectives and challenges for gene therapy. Curr. Gene Ther. 11, 11–27 (2011).
Rosen, L. E. et al. Homing endonuclease I-CreI derivatives with novel DNA target specificities. Nucleic Acids Res. 34, 4791–4800 (2006).
Doyon, J. B., Pattanayak, V., Meyer, C. B. & Liu, D. R. Directed evolution and substrate specificity profile of homing endonuclease I-SceI. J. Am. Chem. Soc. 128, 2477–2484 (2006).
Arnould, S. et al. Engineering of large numbers of highly specific homing endonucleases that induce recombination on novel DNA targets. J. Mol. Biol. 355, 443–458 (2006).
Ashworth, J. et al. Computational redesign of endonuclease DNA binding and cleavage specificity. Nature 441, 656–659 (2006).
Wolfe, S. A., Nekludova, L. & Pabo, C. O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 29, 183–212 (2000).
Pavletich, N. P. & Pabo, C. O. Zinc finger-DNA recognition: crystal structure of a Zif268–DNA complex at 2.1 A. Science 252, 809–817 (1991).
Liu, Q., Segal, D. J., Ghiara, J. B. & Barbas, C. F. 3rd. Design of polydactyl zinc-finger proteins for unique addressing within complex genomes. Proc. Natl Acad. Sci. USA 94, 5525–5530 (1997).
Kim, Y. G., Cha, J. & Chandrasegaran, S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc. Natl Acad. Sci. USA 93, 1156–1160 (1996).
Beerli, R. R., Dreier, B. & Barbas, C. F. 3rd. Positive and negative regulation of endogenous genes by designed transcription factors. Proc. Natl Acad. Sci. USA 97, 1495–1500 (2000).
Akopian, A., He, J., Boocock, M. R. & Stark, W. M. Chimeric recombinases with designed DNA sequence recognition. Proc. Natl Acad. Sci. USA 100, 8688–8691 (2003).
Gordley, R. M., Gersbach, C. A. & Barbas, C. F. 3rd. Synthesis of programmable integrases. Proc. Natl Acad. Sci. USA 106, 5053–5058 (2009).
Kay, S., Hahn, S., Marois, E., Hause, G. & Bonas, U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318, 648–651 (2007).
Romer, P. et al. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 318, 645–648 (2007).
Moscou, M. J. & Bogdanove, A. J. A simple cipher governs DNA recognition by TAL effectors. Science 326, 1501 (2009).
Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512 (2009).
Moore, R., Chandrahas, A. & Bleris, L. Transcription activator-like effectors: a toolkit for synthetic biology. ACS Synth. Biol. 3, 708–716 (2014).
Christian, M. et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186, 757–761 (2010).
Morbitzer, R., Romer, P., Boch, J. & Lahaye, T. Regulation of selected genome loci using de novo-engineered transcription activator-like effector (TALE)-type transcription factors. Proc. Natl Acad. Sci. USA 107, 21617–21622 (2010).
Miller, J. C. et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 29, 143–148 (2011).
Zhang, F. et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat. Biotechnol. 29, 149–153 (2011).
Mercer, A. C., Gaj, T., Fuller, R. P. & Barbas, C. F. 3rd. Chimeric TALE recombinases with programmable DNA sequence specificity. Nucleic Acids Res. 40, 11163–11172 (2012).
Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39, e82 (2011).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Makarova, K. S. et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 9, 467–477 (2011).
Barrangou, R. & Doudna, J. A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 34, 933–941 (2016).
Brouns, S. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Deltcheva, E. et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471, 602–607 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR–Cas9. Science 346, 1258096 (2014).
Zhou, Y. et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature 509, 487–491 (2014).
Koike-Yusa, H., Li, Y., Tan, E. P., Velasco-Herrera Mdel, C. & Yusa, K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat. Biotechnol. 32, 267–273 (2014).
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR–Cas9 system. Science 343, 80–84 (2014).
Thakore, P. I., Black, J. B., Hilton, I. B. & Gersbach, C. A. Editing the epigenome: technologies for programmable transcription and epigenetic modulation. Nat. Methods 13, 127–137 (2016).
Beerli, R. R., Segal, D. J., Dreier, B. & Barbas, C. F. 3rd. Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc. Natl Acad. Sci. USA 95, 14628–14633 (1998).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Maeder, M. L. et al. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 10, 977–979 (2013).
Perez-Pinera, P. et al. RNA-guided gene activation by CRISPR–Cas9-based transcription factors. Nat. Methods 10, 973–976 (2013).
Cong, L., Zhou, R., Kuo, Y. C., Cunniff, M. & Zhang, F. Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. Nat. Commun. 3, 968 (2012).
Thakore, P. I. et al. Highly specific epigenome editing by CRISPR–Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 12, 1143–1149 (2015).
Carvin, C. D., Parr, R. D. & Kladde, M. P. Site-selective in vivo targeting of cytosine-5 DNA methylation by zinc-finger proteins. Nucleic Acids Res. 31, 6493–6501 (2003).
Maeder, M. L. et al. Targeted DNA demethylation and activation of endogenous genes using programmable TALE–TET1 fusion proteins. Nat. Biotechnol. 31, 1137–1142 (2013).
Hilton, I. B. et al. Epigenome editing by a CRISPR–Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 33, 510–517 (2015).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Yu, H. H. et al. Porcine zygote injection with Cas9/sgRNA results in DMD-modified pig with muscle dystrophy. Int. J. Mol. Sci. 17, 1668 (2016).
Chen, Y. et al. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum. Mol. Genet. 24, 3764–3774 (2015).
Palmieri, B. & Tremblay, J. P. Myoblast transplantation: a possible surgical treatment for a severe pediatric disease. Surg. Today 40, 902–908 (2010).
Darabi, R. et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10, 610–619 (2012).
Riolobos, L. et al. HLA engineering of human pluripotent stem cells. Mol. Ther. 21, 1232–1241 (2013).
Li, H. L. et al. Precise correction of the dystrophin gene in Duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR–Cas9. Stem Cell Rep. 4, 143–154 (2015).
Young, C. S. et al. A single CRISPR–Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 18, 533–540 (2016).
Zhao, C. et al. Recombinase-mediated reprogramming and dystrophin gene addition in mdx mouse induced pluripotent stem cells. PLoS ONE 9, e96279 (2014).
Maggio, I., Chen, X. Y. & Goncalves, M. A. The emerging role of viral vectors as vehicles for DMD gene editing. Genome Med. 8, 59 (2016).
Gruber, K. Europe gives gene therapy the green light. Lancet 380, e10 (2012).
Arruda, V. R. et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood 103, 85–92 (2004).
Wang, Z. et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 23, 321–328 (2005).
Inagaki, K. et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 14, 45–53 (2006).
Wu, Z., Asokan, A. & Samulski, R. J. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14, 316–327 (2006).
Asokan, A., Schaffer, D. V. & Samulski, R. J. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. 20, 699–708 (2012).
McCarty, D. M., Young Jr, S. M. & Samulski, R. J. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 38, 819–845 (2004).
Deyle, D. R. & Russell, D. W. Adeno-associated virus vector integration. Curr. Opin. Mol. Ther. 11, 442–447 (2009).
Seto, J. T., Ramos, J. N., Muir, L., Chamberlain, J. S. & Odom, G. L. Gene replacement therapies for duchenne muscular dystrophy using adeno-associated viral vectors. Curr. Gene Ther. 12, 139–151 (2012).
Wu, Z., Yang, H. & Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 18, 80–86 (2010).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Kotterman, M. A. & Schaffer, D. V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445–451 (2014).
Maheshri, N., Koerber, J. T., Kaspar, B. K. & Schaffer, D. V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24, 198–204 (2006).
Li, W. et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol. Ther. 16, 1252–1260 (2008).
Asokan, A. et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 28, 79–82 (2010).
Shen, S. et al. Engraftment of a galactose receptor footprint onto adeno-associated viral capsids improves transduction efficiency. J. Biol. Chem. 288, 28814–28823 (2013).
Yang, L. et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc. Natl Acad. Sci. USA 106, 3946–3951 (2009).
Schmidt, F. & Grimm, D. CRISPR genome engineering and viral gene delivery: a case of mutual attraction. Biotechnol. J. 10, 258–272 (2015).
Nelson, C. E. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016).
Tabebordbar, M. et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411 (2016).
Long, C. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403 (2016).
Lombardo, A. et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 25, 1298–1306 (2007).
Persons, D. A. Lentiviral vector gene therapy: effective and safe? Mol. Ther. 18, 861–862 (2010).
Kabadi, A. M., Ousterout, D. G., Hilton, I. B. & Gersbach, C. A. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 42, e147 (2014).
Izmiryan, A., Basmaciogullari, S., Henry, A., Paques, F. & Danos, O. Efficient gene targeting mediated by a lentiviral vector-associated meganuclease. Nucleic Acids Res. 39, 7610–7619 (2011).
Cai, Y., Bak, R. O. & Mikkelsen, J. G. Targeted genome editing by lentiviral protein transduction of zinc-finger and TAL-effector nucleases. eLife 3, e01911 (2014).
Holkers, M. et al. Differential integrity of TALE nuclease genes following adenoviral and lentiviral vector gene transfer into human cells. Nucleic Acids Res. 41, e63 (2013).
Wang, D. et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum. Gene Ther. 26, 432–442 (2015).
Raper, S. E. et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80, 148–158 (2003).
Geutskens, S. B. et al. Recombinant adenoviral vectors have adjuvant activity and stimulate T cell responses against tumor cells. Gene Ther. 7, 1410–1416 (2000).
Glover, D. J., Lipps, H. J. & Jans, D. A. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 6, 299–310 (2005).
Nelson, C. E. & Gersbach, C. A. Engineering delivery vehicles for genome editing. Annu. Rev. Chem. Biomol. Eng. 7, 637–662 (2016).
Yin, H. et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555 (2014).
Wooddell, C. I. et al. Dose response in rodents and nonhuman primates after hydrodynamic limb vein delivery of naked plasmid DNA. Hum. Gene Ther. 22, 889–903 (2011).
Zhang, G. et al. Functional efficacy of dystrophin expression from plasmids delivered to mdx mice by hydrodynamic limb vein injection. Hum. Gene Ther. 21, 221–237 (2010).
Lu, Q. L., Bou-Gharios, G. & Partridge, T. A. Non-viral gene delivery in skeletal muscle: a protein factory. Gene Ther. 10, 131–142 (2003).
Yin, H. et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat. Biotechnol. 32, 551–553 (2014).
Rando, T. A. Non-viral gene therapy for Duchenne muscular dystrophy: progress and challenges. Biochim. Biophys. Acta 1772, 263–271 (2007).
McNeer, N. A. et al. Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene Ther. 20, 658–669 (2013).
Zuris, J. A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 33, 73–80 (2015).
Long, C., Amoasii, L., Bassel-Duby, R. & Olson, E. N. Genome editing of monogenic neuromuscular diseases: a systematic review. JAMA Neurol. 73, 1349–1355 (2016).
Robinson-Hamm, J. N. & Gersbach, C. A. Gene therapies that restore dystrophin expression for the treatment of Duchenne muscular dystrophy. Hum. Genet. 135, 1029–1040 (2016).
Davies, K. E. & Nowak, K. J. Molecular mechanisms of muscular dystrophies: old and new players. Nat. Rev. Mol. Cell Biol. 7, 762–773 (2006).
Davie, A. M. & Emery, A. E. Estimation of proportion of new mutants among cases of Duchenne muscular dystrophy. J. Med. Genet. 15, 339–345 (1978).
Bushby, K. et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 9, 77–93 (2010).
Larkindale, J. et al. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve 49, 431–438 (2014).
Wang, B., Li, J. & Xiao, X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl Acad. Sci. USA 97, 13714–13719 (2000).
Liu, M. et al. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol. Ther. 11, 245–256 (2005).
Zhang, Y. & Duan, D. Novel mini–dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum. Gene Ther. 23, 98–103 (2012).
Aartsma-Rus, A. et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 30, 293–299 (2009).
Goyenvalle, A. et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306, 1796–1799 (2004).
Mendell, J. R. et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 79, 257–271 (2016).
Goyenvalle, A. et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat. Med. 21, 270–275 (2015).
Chapdelaine, P., Pichavant, C., Rousseau, J., Paques, F. & Tremblay, J. P. Meganucleases can restore the reading frame of a mutated dystrophin. Gene Ther. 17, 846–858 (2010).
Popplewell, L. et al. Gene correction of a Duchenne muscular dystrophy mutation by meganuclease-enhanced exon knock-in. Hum. Gene Ther. 24, 692–701 (2013).
Ousterout, D. G. et al. Correction of dystrophin expression in cells from Duchenne muscular dystrophy patients through genomic excision of exon 51 by zinc finger nucleases. Mol. Ther. 23, 523–532 (2015).
Ousterout, D. G. et al. Reading frame correction by targeted genome editing restores dystrophin expression in cells from Duchenne muscular dystrophy patients. Mol. Ther. 21, 1718–1726 (2013).
Ousterout, D. G. et al. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun. 6, 6244 (2015).
Maggio, I. et al. Selection-free gene repair after adenoviral vector transduction of designer nucleases: rescue of dystrophin synthesis in DMD muscle cell populations. Nucleic Acids Res. 44, 1449–1470 (2016).
Iyombe-Engembe, J. P. et al. Efficient restoration of the dystrophin gene reading frame and protein structure in DMD myoblasts using the CinDel method. Mol. Ther. Nucleic Acids 5, e283 (2016).
Xu, L. et al. CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol. Ther. 24, 564–569 (2015).
Bengtsson, N. E. et al. Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat. Commun. 8, 14454 (2017).
Hartmann, J. & Croteau, S. E. 2017 clinical trials update: innovations in hemophilia therapy. Am. J. Hematol. 91, 1252–1260 (2016).
Suzuki, K. et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 540, 144–149 (2016).
Kasparek, P. et al. Efficient gene targeting of the Rosa26 locus in mouse zygotes using TALE nucleases. FEBS Lett. 588, 3982–3988 (2014).
Remy, S. et al. Efficient gene targeting by homology-directed repair in rat zygotes using TALE nucleases. Genome Res. 24, 1371–1383 (2014).
Sato, T. et al. Genome editing in mouse spermatogonial stem cell lines using TALEN and double-nicking CRISPR/Cas9. Stem Cell Rep. 5, 75–82 (2015).
Quadros, R. M., Harms, D. W., Ohtsuka, M. & Gurumurthy, C. B. Insertion of sequences at the original provirus integration site of mouse ROSA26 locus using the CRISPR/Cas9 system. FEBS Open Bio 5, 191–197 (2015).
Benabdallah, B. F. et al. Targeted gene addition of microdystrophin in mice skeletal muscle via human myoblast transplantation. Mol. Ther. Nucleic Acids 2, e68 (2013).
Tinsley, J., Robinson, N. & Davies, K. E. Safety, tolerability, and pharmacokinetics of SMT C1100, a 2-arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to healthy male adult volunteers. J. Clin. Pharmacol. 55, 698–707 (2015).
Corbi, N. et al. The artificial zinc finger coding gene 'Jazz' binds the utrophin promoter and activates transcription. Gene Ther. 7, 1076–1083 (2000).
Di Certo, M. G. et al. The artificial gene Jazz, a transcriptional regulator of utrophin, corrects the dystrophic pathology in mdx mice. Hum. Mol. Genet. 19, 752–760 (2010).
Strimpakos, G. et al. Novel adeno-associated viral vector delivering the utrophin gene regulator Jazz counteracts dystrophic pathology in mdx mice. J. Cell. Physiol. 229, 1283–1291 (2014).
Onori, A. et al. The artificial 4-zinc-finger protein Bagly binds human utrophin promoter A at the endogenous chromosomal site and activates transcription. Biochem. Cell Biol. 85, 358–365 (2007).
Onori, A. et al. UtroUp is a novel six zinc finger artificial transcription factor that recognises 18 base pairs of the utrophin promoter and efficiently drives utrophin upregulation. BMC Mol. Biol. 14, 3 (2013).
Foust, K. D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 (2009).
Duque, S. et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 17, 1187–1196 (2009).
Foust, K. D. et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 28, 271–274 (2010).
DiMatteo, D., Callahan, S. & Kmiec, E. B. Genetic conversion of an SMN2 gene to SMN1: a novel approach to the treatment of spinal muscular atrophy. Exp. Cell Res. 314, 878–886 (2008).
Corti, S. et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci. Transl Med. 4, 165ra162 (2012).
Passini, M. A. et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl Med. 3, 72ra18 (2011).
Porensky, P. N. et al. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 21, 1625–1638 (2012).
Wijmenga, C. et al. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet 336, 651–653 (1990).
van Deutekom, J. C. et al. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum. Mol. Genet. 2, 2037–2042 (1993).
Gabellini, D., Green, M. R. & Tupler, R. Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell 110, 339–348 (2002).
Lemmers, R. J. et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 329, 1650–1653 (2010).
van Overveld, P. G. et al. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat. Genet. 35, 315–317 (2003).
Lek, A., Rahimov, F., Jones, P. L. & Kunkel, L. M. Emerging preclinical animal models for FSHD. Trends Mol. Med. 21, 295–306 (2015).
Tupler, R. et al. Monosomy of distal 4q does not cause facioscapulohumeral muscular dystrophy. J. Med. Genet. 33, 366–370 (1996).
Tawil, R. & Van Der Maarel, S. M. Facioscapulohumeral muscular dystrophy. Muscle Nerve 34, 1–15 (2006).
Wallace, L. M. et al. DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann. Neurol. 69, 540–552 (2011).
Pandey, S. N., Lee, Y. C., Yokota, T. & Chen, Y. W. Morpholino treatment improves muscle function and pathology of Pitx1 transgenic mice. Mol. Ther. 22, 390–396 (2014).
Gabellini, D. et al. Facioscapulohumeral muscular dystrophy in mice overexpressing FRG1. Nature 439, 973–977 (2006).
Feeney, S. J. et al. FHL1 reduces dystrophy in transgenic mice overexpressing FSHD muscular dystrophy region gene 1 (FRG1). PLoS ONE 10, e0117665 (2015).
Bortolanza, S. et al. AAV6-mediated systemic shRNA delivery reverses disease in a mouse model of facioscapulohumeral muscular dystrophy. Mol. Ther. 19, 2055–2064 (2011).
Wallace, L. M. et al. RNA interference inhibits DUX4-induced muscle toxicity in vivo: implications for a targeted FSHD therapy. Mol. Ther. 20, 1417–1423 (2012).
Himeda, C. L., Jones, T. I. & Jones, P. L. CRISPR/dCas9-mediated transcriptional inhibition ameliorates the epigenetic dysregulation at D4Z4 and represses DUX4-fl in FSH muscular dystrophy. Mol. Ther. 24, 527–535 (2015).
Richard, G. F. Shortening trinucleotide repeats using highly specific endonucleases: a possible approach to gene therapy? Trends Genet. 31, 177–186 (2015).
Godinho, B. M., Malhotra, M., O'Driscoll, C. M. & Cryan, J. F. Delivering a disease-modifying treatment for Huntington's disease. Drug Discov. Today 20, 50–64 (2015).
Richard, G. F., Dujon, B. & Haber, J. E. Double-strand break repair can lead to high frequencies of deletions within short CAG/CTG trinucleotide repeats. Mol. Gen. Genet. 261, 871–882 (1999).
Mittelman, D. et al. Zinc-finger directed double-strand breaks within CAG repeat tracts promote repeat instability in human cells. Proc. Natl Acad. Sci. USA 106, 9607–9612 (2009).
Garriga-Canut, M. et al. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc. Natl Acad. Sci. USA 109, E3136–E3145 (2012).
Richard, G. F. et al. Highly specific contractions of a single CAG/CTG trinucleotide repeat by TALEN in yeast. PLoS ONE 9, e95611 (2014).
De Michele, G. & Filla, A. Movement disorders: Friedreich ataxia today — preparing for the final battle. Nat. Rev. Neurol. 11, 188–190 (2015).
Campuzano, V. et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 (1996).
Gerard, C. et al. An AAV9 coding for frataxin clearly improved the symptoms and prolonged the life of Friedreich ataxia mouse models. Mol. Ther. Methods Clin. Dev. 1, 14044 (2014).
Chapdelaine, P., Coulombe, Z., Chikh, A., Gerard, C. & Tremblay, J. P. A potential new therapeutic approach for Friedreich ataxia: induction of frataxin expression with TALE proteins. Mol. Ther. Nucleic Acids 2, e119 (2013).
Li, Y. J. et al. Excision of expanded GAA repeats alleviates the molecular phenotype of Friedreich's ataxia. Mol. Ther. 23, 1055–1065 (2015).
Victor, M., Hayes, R. & Adams, R. D. Oculopharyngeal muscular dystrophy. A familial disease of late life characterized by dysphagia and progressive ptosis of the eyelids. N. Engl. J. Med. 267, 1267–1272 (1962).
Brais, B. et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat. Genet. 18, 164–167 (1998).
Brais, B. et al. The oculopharyngeal muscular dystrophy locus maps to the region of the cardiac alpha and beta myosin heavy chain genes on chromosome 14q11.2–q13. Hum. Mol. Genet. 4, 429–434 (1995).
Ravikumar, B., Duden, R. & Rubinsztein, D. C. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 11, 1107–1117 (2002).
Abu-Baker, A. & Rouleau, G. A. Oculopharyngeal muscular dystrophy: recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochim. Biophys. Acta 1772, 173–185 (2007).
Davies, J. E., Sarkar, S. & Rubinsztein, D. C. Trehalose reduces aggregate formation and delays pathology in a transgenic mouse model of oculopharyngeal muscular dystrophy. Hum. Mol. Genet. 15, 23–31 (2006).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02015481 (2016).
Perie, S. et al. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol. Ther. 22, 219–225 (2014).
Harish, P., Malerba, A., Dickson, G. & Bachtarzi, H. Progress on gene therapy, cell therapy, and pharmacological strategies toward the treatment of oculopharyngeal muscular dystrophy. Hum. Gene Ther. 26, 286–292 (2015).
Shelbourne, P. & Johnson, K. Myotonic dystrophy: another case of too many repeats? Hum. Mutat. 1, 183–189 (1992).
Mahadevan, M. et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Harley, H. G. et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature 355, 545–546 (1992).
Brook, J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Udd, B. & Krahe, R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 11, 891–905 (2012).
Xia, G. et al. Genome modification leads to phenotype reversal in human myotonic dystrophy type 1 induced pluripotent stem cell-derived neural stem cells. Stem Cells 33, 1829–1838 (2015).
Zhang, W. et al. Treatment of type 1 myotonic dystrophy by engineering site-specific RNA endonucleases that target (CUG)n repeats. Mol. Ther. 22, 312–320 (2014).
Krahn, M. et al. A naturally occurring human minidysferlin protein repairs sarcolemmal lesions in a mouse model of dysferlinopathy. Sci. Transl Med. 2, 50ra69 (2010).
Sondergaard, P. C. et al. AAV.dysferlin overlap vectors restore function in dysferlinopathy animal models. Ann. Clin. Transl Neurol. 2, 256–270 (2015).
Lostal, W. et al. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum. Mol. Genet. 19, 1897–1907 (2010).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02710500 (2016).
Tedesco, F. S. et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci. Transl Med. 4, 140ra189 (2012).
Turan, S., Farruggio, A. P., Srifa, W., Day, J. W. & Calos, M. P. Precise correction of disease mutations in induced pluripotent stem cells derived from patients with limb girdle muscular dystrophy. Mol. Ther. 24, 685–696 (2016).
Liu, J. & Harper, S. Q. RNAi-based gene therapy for dominant limb girdle muscular dystrophies. Curr. Gene Ther. 12, 307–314 (2012).
Liu, J. et al. RNAi-mediated gene silencing of mutant myotilin improves myopathy in LGMD1A mice. Mol. Ther. Nucleic Acids 3, e160 (2014).
Cohn, R. D. & Campbell, K. P. Molecular basis of muscular dystrophies. Muscle Nerve 23, 1456–1471 (2000).
Oldfors, A. Hereditary myosin myopathies. Neuromuscul. Disord. 17, 355–367 (2007).
Schroder, R. & Schoser, B. Myofibrillar myopathies: a clinical and myopathological guide. Brain Pathol. 19, 483–492 (2009).
Udd, B. Distal myopathies — new genetic entities expand diagnostic challenge. Neuromuscul. Disord. 22, 5–12 (2012).
Udd, B. Distal myopathies. Curr. Neurol. Neurosci. Rep. 14, 434 (2014).
Kemaladewi, D. U. et al. Correction of a splicing defect in a mouse model of congenital muscular dystrophy type 1A using a homology-directed-repair-independent mechanism. Nat. Med. 23, 984–989 (2017).
Vannoy, C. H. et al. Adeno-associated virus-mediated overexpression of LARGE rescues α-dystroglycan function in dystrophic mice with mutations in the fukutin-related protein. Hum. Gene Ther. Methods 25, 187–196 (2014).
Yu, M. et al. Adeno-associated viral-mediated LARGE gene therapy rescues the muscular dystrophic phenotype in mouse models of dystroglycanopathy. Hum. Gene Ther. 24, 317–330 (2013).
Xu, L., Zhao, P., Mariano, A. & Han, R. Targeted myostatin gene editing in multiple mammalian species directed by a single pair of TALE nucleases. Mol. Ther. Nucleic Acids 2, e112 (2013).
Buckland, K. F. & Bobby Gaspar, H. Gene and cell therapy for children — new medicines, new challenges? Adv. Drug Deliv. Rev. 73, 162–169 (2014).
Arnett, A. L. et al. Adeno-associated viral vectors do not efficiently target muscle satellite cells. Mol. Ther. Methods Clin. Dev. 1, 14038 (2014).
Stitelman, D. H. et al. Developmental stage determines efficiency of gene transfer to muscle satellite cells by in utero delivery of adeno-associated virus vector serotype 2/9. Mol. Ther. Methods Clin. Dev. 1, 14040 (2014).
Kimura, E., Li, S., Gregorevic, P., Fall, B. M. & Chamberlain, J. S. Dystrophin delivery to muscles of mdx mice using lentiviral vectors leads to myogenic progenitor targeting and stable gene expression. Mol. Ther. 18, 206–213 (2010).
Tsai, S. Q. et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR–Cas nucleases. Nat. Biotechnol. 33, 187–197 (2015).
Shen, B. et al. Efficient genome modification by CRISPR–Cas9 nickase with minimal off-target effects. Nat. Methods 11, 399–402 (2014).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Kleinstiver, B. P. et al. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Mingozzi, F. & High, K. A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122, 23–36 (2013).
Mingozzi, F. et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13, 419–422 (2007).
Mingozzi, F. et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl Med. 5, 194ra192 (2013).
Madsen, D., Cantwell, E. R., O'Brien, T., Johnson, P. A. & Mahon, B. P. Adeno-associated virus serotype 2 induces cell-mediated immune responses directed against multiple epitopes of the capsid protein VP1. J. Gen. Virol. 90, 2622–2633 (2009).
Faust, S. M. et al. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Invest. 123, 2994–3001 (2013).
Wu, T. L. et al. CD8+ T cell recognition of epitopes within the capsid of adeno-associated virus 8-based gene transfer vectors depends on vectors' genome. Mol. Ther. 22, 42–51 (2014).
Chew, W. L. et al. A multifunctional AAV-CRISPR–Cas9 and its host response. Nat. Methods 13, 868–874 (2016).
Mendell, J. R. et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 363, 1429–1437 (2010).
Acknowledgements
The authors' work has been supported by the Muscular Dystrophy Association (grant MDA277360), a Duke–Coulter Translational Partnership Grant, a Duke/UNC-Chapel Hill CTSA Consortium Collaborative Translational Research Award, a Hartwell Foundation Individual Biomedical Research Award, a March of Dimes Foundation Basil O'Connor Starter Scholar Award, NIH grants R01AR069085 and UH3TR000505, an NIH Director's New Innovator Award (DP2-OD008586), the Office of the Assistant Secretary of Defense for Health Affairs, through the Duchenne Muscular Dystrophy Research Program under awards W81XWH-15-1-0469 and W81XWH-16-1-0221, the Thorek Memorial Foundation, and the Allen Distinguished Investigator Program, through The Paul G. Allen Frontiers Group. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the NIH or Department of Defense. C.E.N. is supported by a Hartwell Foundation Postdoctoral Fellowship and J.R.H. is supported by a National Science Foundation Graduate Research Fellowship and American Heart Association Predoctoral Fellowship.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.A.G., C.E.N. and J.R.H. are inventors on patent applications related to genome engineering for neuromuscular diseases.
Glossary
- Exon skipping
-
A technique in which antisense oligonucleotides are introduced that bind motifs within an mRNA, promoting alternative splicing and excluding the targeted exon from the final transcript.
- Nonsense readthrough compounds
-
Small molecules that suppress stop codon recognition and allow ribosomes to translate an mRNA, despite nonsense mutations.
- Morpholino
-
A non-ionic synthetic nucleic acid with DNA bases covalently attached to morpholine rings with a phosphorodiamidate backbone.
- Homology-directed repair
-
(HDR). A precise method of DNA repair that uses the sister chromosome or a provided DNA template to restore the damaged genome or introduce new DNA sequences.
- Safe-harbour locus
-
A genomic region where targeted integrations can be performed without disrupting endogenous gene activity or negatively affecting cell function.
- Non-homologous end joining
-
(NHEJ). A DNA repair pathway that religates double-strand breaks, with the possibility of producing small insertions or deletions that might lead to gene knockout.
- Directed evolution
-
A process that uses multiple rounds of mutagenesis and user-defined selection to alter the function of a protein or nucleic acid.
- Cys2–His2 zinc finger domain
-
A finger-like structure of ∼30 amino acids that recognizes three or four base pairs. Proteins containing multiple Cys2–His2 zinc finger domains can be used for sequence-specific DNA targeting.
- Golden Gate assembly
-
A cloning method that allows scarless ligation of multiple DNA fragments and is well-suited for cloning repetitive sequences, including TALE arrays.
- Protospacer adjacent motif
-
(PAM). A sequence motif that is recognized by the Cas9 enzyme. Several CRISPR systems have been described with varying PAM requirements.
- Small interfering RNA
-
Short, double-stranded RNA that mediates sequence-specific silencing of targeted mRNAs.
Rights and permissions
About this article
Cite this article
Nelson, C., Robinson-Hamm, J. & Gersbach, C. Genome engineering: a new approach to gene therapy for neuromuscular disorders. Nat Rev Neurol 13, 647–661 (2017). https://doi.org/10.1038/nrneurol.2017.126
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.126
This article is cited by
-
A biallelic variant of DCAF13 implicated in a neuromuscular disorder in humans
European Journal of Human Genetics (2023)
-
Engineering the next generation of cell-based therapeutics
Nature Reviews Drug Discovery (2022)
-
Duchenne muscular dystrophy
Nature Reviews Disease Primers (2021)
-
A glance at genome editing with CRISPR–Cas9 technology
Current Genetics (2020)
-
A rocky road for the maturation of embryo-editing methods
Nature Methods (2019)