Key Points
-
Early and accurate diagnosis of Creutzfeldt–Jakob disease (CJD) is essential to avoid iatrogenic transmission and to distinguish CJD from potentially treatable dementias
-
Diagnosis of CJD in living patients is challenging, mainly because the disease phenotypes are highly heterogeneous, and detection of the misfolded protein in the brain tissue is often not feasible
-
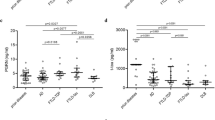
Supportive investigations such as EEG, MRI and cerebrospinal fluid biomarkers have a relatively low diagnostic sensitivity and specificity in CJD
-
Diagnosis of CJD has been markedly improved by novel ultrasensitive seeding assays, such as real-time quaking-induced conversion (RT-QuIC) and protein misfolding cyclic amplification (PMCA), which are based on amplified prion detection
-
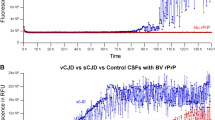
RT-QuIC is specific and highly sensitive for sporadic CJD, whereas PMCA is extremely sensitive for detecting variant CJD prions in biological fluids and in extraneural or lymphatic tissues
-
In the future, novel assays analogous to RT-QuIC or PMCA might provide a protein-seeding-based diagnosis in other neurodegenerative diseases in which prion-like neurodegenerative processes are implicated
Abstract
Early and accurate diagnosis of Creutzfeldt–Jakob disease (CJD) is a necessary to distinguish this untreatable disease from treatable rapidly progressive dementias, and to prevent iatrogenic transmission. Currently, definitive diagnosis of CJD requires detection of the abnormally folded, CJD-specific form of protease-resistant prion protein (PrPCJD) in brain tissue obtained postmortem or via biopsy; therefore, diagnosis of sporadic CJD in clinical practice is often challenging. Supporting investigations, including MRI, EEG and conventional analyses of cerebrospinal fluid (CSF) biomarkers, are helpful in the diagnostic work-up, but do not allow definitive diagnosis. Recently, novel ultrasensitive seeding assays, based on the amplified detection of PrPCJD, have improved the diagnostic process; for example, real-time quaking-induced conversion (RT-QuIC) is a sensitive method to detect prion-seeding activity in brain homogenate from humans with any subtype of sporadic CJD. RT-QuIC can also be used for in vivo diagnosis of CJD: its diagnostic sensitivity in detecting PrPCJD in CSF samples is 96%, and its specificity is 100%. Recently, we provided evidence that RT-QuIC of olfactory mucosa brushings is a 97% sensitive and 100% specific for sporadic CJD. These assays provide a basis for definitive antemortem diagnosis of prion diseases and, in doing so, improve prospects for reducing the risk of prion transmission. Moreover, they can be used to evaluate outcome measures in therapeutic trials for these as yet untreatable infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
14 June 2016
In the version of this article initially published online and in print, the Figure 2 erroneously depicted recombinant prion protein with attached glycans, and Table 2 listed an erroneous glycotype for sporadic Creutzfeldt–Jakob disease. The errors have been corrected for the PDF and HTML versions of the article.
References
Aguzzi, A. O'Connor, T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 9, 237–248 (2010).
Kraus, A., Groveman, B. R. & Caughey, B. Prions and the potential transmissibility of protein misfolding diseases. Annu. Rev. Microbiol. 67, 543–564 (2013).
Zou, W. Q. et al. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann. Neurol. 68, 162–172 (2010).
Parchi, P. et al. A subtype of sporadic prion disease mimicking fatal familial insomnia. Neurology 52, 1757–1763 (1999).
Lugaresi, E. et al. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N. Engl. J. Med. 315, 997–1003 (1986).
Piccardo, P. et al. Phenotypic variability of Gerstmann–Sträussler–Scheinker disease is associated with prion protein heterogeneity. J. Neuropathol. Exp. Neurol. 57, 979–988 (1998).
Mead, S. et al. A novel prion disease associated with diarrhea and autonomic neuropathy. N. Engl. J. Med. 369, 1904–1914 (2013).
Mead, S. & Reilly, M. M. A new prion disease: relationship with central and peripheral amyloidoses. Nat. Rev. Neurol. 11, 90–97 (2015).
Geschwind, M. D., Shu, H., Haman, A., Sejvar, J. J. & Miller, B. L. Rapidly progressive dementia. Ann. Neurol. 64, 97–108 (2008).
Chitravas, N. et al. Treatable neurological disorders misdiagnosed as Creutzfeldt–Jakob disease. Ann. Neurol. 70, 437–444 (2011).
Paterson, R. W. et al. Differential diagnosis of Jakob–Creutzfeldt disease. Arch. Neurol. 69, 1578–1582 (2012).
Collins, S. J. et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt–Jakob disease. Brain 129, 2278–2287 (2006).
Brown, P. & Farrell, M. A practical approach to avoiding iatrogenic Creutzfeldt–Jakob disease (CJD) from invasive instruments. Infect. Control Hosp. Epidemiol. 36, 844–848 (2015).
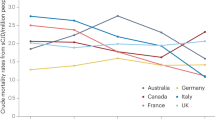
Ladogana, A. et al. Mortality from Creutzfeldt–Jakob disease and related disorders in Europe, Australia, and Canada. Neurology 64, 1586–1591 (2005).
Diack, A. B. et al. Variant CJD. 18 years of research and surveillance. Prion 8, 286–295 (2014).
Gambetti, P., Kong, Q., Zou, W., Parchi, P. & Chen, S. G. Sporadic and familial CJD: classification and characterisation. Br. Med. Bull. 66, 213–239 (2003).
Parchi, P. et al. Classification of sporadic Creutzfeldt–Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 46, 224–233 (1999).
Pocchiari, M. Predictors of survival in sporadic Creutzfeldt–Jakob disease and other human transmissible spongiform encephalopathies. B rain 127, 2348–2359 (2004).
Puoti, G. et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 11, 618–628 (2012).
Alperovitch, A. et al. Codon 129 prion protein genotype and sporadic Creutzfeldt–Jakob disease. Lancet 353, 1673–1674 (1999).
Mead, S. et al. Balancing selection at the prion protein gene consistent with prehistoric kuru-like epidemics. Science 300, 640–643 (2003).
Parchi, P. et al. Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol. 124, 517–529 (2012).
Heath, C. A. et al. Validation of diagnostic criteria for variant Creutzfeldt–Jakob disease. Ann. Neurol. 67, 761–770 (2010).
Binelli, S. et al. Periodic electroencephalogram complexes in a patient with variant Creutzfeldt–Jakob disease. Ann. Neurol. 59, 423–427 (2006).
Hill, A. F. et al. Investigation of variant Creutzfeldt–Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353, 183–189 (1999).
Peden, A. H. et al. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364, 527–529 (2004).
Peden, A. et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia 16, 296–304 (2010).
Bishop, M. T. et al. Prion infectivity in the spleen of a PRNP heterozygous individual with subclinical variant Creutzfeldt–Jakob disease. Brain 136, 1139–1145 (2013).
Gill, O. N. et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ 347, 5675f (2013). A major analysis of 32,441 appendix samples for abnormal prion protein as a marker of vCJD carrier status, indicating an overall prevalence of 493 per 1,000,000.
Gillies, M. et al. A retrospective case note review of deceased recipients of vCJD-implicated blood transfusions. Vox Sang. 97, 211–218 (2009).
Wallis, J. P. Strategies to reduce transfusion acquired vCJD. Transfus. Med. 21, 1–6 (2011).
Zerr, I. et al. Updated clinical diagnostic criteria for sporadic Creutzfeld–Jakob disease. Brain 132, 2659–2668 (2009).
Zerr, I. et al. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt–Jakob disease. Neurology 55, 811–815 (2000).
Meissner, B. et al. MRI lesion profiles in sporadic Creutzfeldt–Jakob disease. Neurology 72, 1994–2001 (2009).
Vitali, P. et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology 76, 1711–1719 (2011).
Satoh, J., Kurohara, K., Yukitake, M. & Kuroda, Y. The 14-3-3 protein detectable in the cerebrospinal fluid of patients with prion-unrelated neurological diseases is expressed constitutively in neurons and glial cells in culture. Eur. Neurol. 41, 216–225 (1999).
Zanusso, G. et al. Phosphorylated 14-3-3ζ protein in the CSF of neuroleptic-treated patients. Neurology 64, 1618–1620 (2005).
Zanusso, G. et al. Cerebrospinal fluid markers in sporadic Creutzfeldt–Jakob disease. Int. J. Mol. Sci. 12, 6281–6292 (2011).
Brown, P. et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann. Neurol. 35, 513–529 (1994).
Douet, J. Y. et al. Detection of infectivity in blood of persons with variant and sporadic Creutzfeldt–Jakob disease. Emerg. Infect. Dis. 20, 114–117 (2014). A seminal demonstration of prion infectivity in the blood of patients with CJD.
Glatzel, M. et al. Extraneural pathologic prion protein in sporadic Creutzfeldt–Jakob disease. N. Engl. J. Med. 349, 1812–1820 (2003).
Peden, A. H. et al. Detection and localization of PrPSc in the skeletal muscle of patients with variant, iatrogenic, and sporadic forms of Creutzfeldt–Jakob disease. Am. J. Pathol. 168, 927–935 (2006).
Rubenstein, R. & Chang, B. Re-assessment of PrPSc distribution in sporadic and variant CJD. PLoS ONE 8, e66352 (2013).
Kovacs, G. G. et al. Creutzfeldt–Jakob disease and inclusion body myositis: abundant disease-associated prion protein in muscle. Ann. Neurol. 55, 121–125 (2004).
Zanusso, G. et al. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt–Jakob disease. N. Engl. J. Med. 348, 711–719 (2003).
Tabaton, M. et al. Prion deposition in olfactory biopsy of sporadic Creutzfeldt–Jakob disease. Ann. Neurol. 55, 294–296 (2004).
Edgeworth, J. A. et al. Detection of prion infection in variant Creutzfeldt–Jakob disease: a blood-based assay. Lancet 377, 487–493 (2011).
Jackson, G. S. et al. A highly specific blood test for vCJD. Blood 123, 452–453 (2014). This study indicated that a blood test, based on prion capture on steel powder coupled with an immunodetection assay, has 100% diagnostic specificity and 70% sensitivity for vCJD.
Raymond, G. J. et al. Molecular assessment of the potential transmissibilities of BSE and scrapie to humans. Nature 388, 285–288 (1997).
Jones, M. et al. In vitro amplification and detection of variant Creutzfeldt–Jakob disease PrPSc. J. Pathol. 213, 21–26 (2007).
Moda, F. et al. Prions in the urine of patients with variant Creutzfeldt–Jakob disease. N. Engl. J. Med. 371, 530–539 (2014). This study demonstrated that PMCA amplification enables detection of PrPCJD in the urine of patients with vCJD, thereby enabling large-scale screening for vCJD to reduce the potential risk of iatrogenic CJD.
Lacroux, C. et al. Preclinical detection of variant CJD and BSE prions in blood. PLoS Pathog. 10, e1004202 (2014).
Oshita, M. et al. Efficient propagation of variant Creutzfeldt–Jakob disease prion protein using the cell-protein misfolding cyclic amplification technique with samples containing plasma and heparin. Transfusion 56, 223–230 (2016).
Castilla, J., Saá, P., Hetz, C. & Soto, C. In vitro generation of infectious scrapie prions. Cell 121, 195–206 (2005).
Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 17, 175–178 (2011). The first demonstration of the utility of RT-QuIC in detection of a disease-specific CJD marker in the CSF, paving the way for reliable diagnosis of living patients with CJD.
McGuire, L. I. et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt–Jakob disease. Ann. Neurol. 72, 278–285 (2012).
Sano, K. et al. Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PLoS ONE 8, e54915 (2013).
Cramm, M. et al. Stability and reproducibility underscore utility of RT-QuIC for diagnosis of Creutzfeldt–Jakob disease. Mol. Neurobiol. 53, 1896–1904 (2016).
Cramm, M. et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol. Neurobiol. 51, 396–405 (2015).
Orrú, C. D. et al. Rapid and sensitive RT-QuIC detection of human Creutzfeldt–Jakob disease using cerebrospinal fluid. mBio 6, e02451–e02414 (2015). A demonstration of the fastest and most sensitive RT-QuIC assay conditions for CSF-based diagnosis of CJD that have been described to date.
Orrú, C. D. et al. A test for Creutzfeldt–Jakob disease using nasal brushings. N. Engl. J. Med. 371, 519–529 (2014). An innovative approach for in vivo sCJD diagnosis: samples of the olfactory mucosa are easily obtained by nasal brushing and are analysed by RT-QuIC, providing 100% specificity and 97% sensitivity.
Zanusso, G., Bongianni, M. & Caughey, B. A test for Creutzfeldt–Jakob disease using nasal brushings. N. Engl. J. Med. 37, 1842–1843 (2014).
Orrú, C. D. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. mBio 2, e00078–00011 (2011).
Orrú, C. D. et al. Bank vole prion protein as an apparently universal substrate for RT-QuIC-based detection and discrimination of prion strains. PLoS Pathog. 11, e1004983 (2015). This recent study demonstrated that a recombinant prion protein substrate for RT-QuIC assays enables detection of at least 28 different prions of humans and other mammals, including several human prion diseases that were not previously detectable by RT-QuIC or PMCA.
Wilham, J. M. et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 6, e1001217 (2010).
Salvadores, N. et al. Detection of misfolded Aβ oligomers for sensitive biochemical diagnosis of Alzheimer's disease. Cell Rep. 7, 261–268 (2014).
Attems, J., Walker, L. & Jellinger, K. A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 127, 459–475 (2014).
Caughey, B. & Baron, G. S. Prions and their partners in crime. Nature 443, 803–810 (2006).
Linden, R. et al. Physiology of the prion protein. Physiol. Rev. 88, 673–728 (2008).
Linden, R., Cordeiro, Y. & Lima, L. M. Allosteric function and dysfunction of the prion protein. Cell. Mol. Life Sci. 69, 1105–1124 (2012).
Laurén, J. et al. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009).
Striebel, J. F., Race, B. & Chesebro, B. Prion protein and susceptibility to kainate-induced seizures: genetic pitfalls in the use of PrP knockout mice. Prion 7, 280–285 (2013).
Brown, P. et al. Iatrogenic Creutzfeldt–Jakob disease, final assessment. Emerg. Infect. Dis. 18, 901–907 (2012).
Acknowledgements
We thank the E-learning facility of the University of Verona for producing the olfactory brushing video. This work was funded in part by Joint Programming Neurodegenerative Disease BIOMARKPD (G.Z.), Fondazione Cariverona “Biomarcatori predittivi e diagnostici in patologie neoplastiche, infiammatorie e neurodegenerative” (G.Z., S.M.) and the Intramural Research Program of the NIAID (B.C.).
Author information
Authors and Affiliations
Contributions
G.Z. and B.C. researched literature for the article and provided substantial contributions to discussion of content. All authors participated in writing of the article and in reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.C. is an inventor on patents and patent applications related to RT-QuIC assays. The other authors declare no competing interests.
Related links
FURTHER INFORMATION
Supplementary information
Supplementary information S1 (video)
This video shows the procedure for obtaining olfactory mucosa samples with nasal brushing. (AVI 883721 kb)
Glossary
- PrPCJD
-
The infectious, often partially protease-resistant form of prion protein that is associated with Creutzfeldt–Jakob disease.
- Prion paradigm
-
According to the prion paradigm, a misfolded, usually pathological, form of a host protein acts as an infectious agent by servingas a folding template, inducing the conversion of its normal counterpart into more of the misfolded form; the misfolded proteins then propagate within and/or between hosts to cause phenotypic changes, such as neurodegenerative disease.
- 14-3-3 protein
-
A protein biomarker of neuronal damage that is not disease-specific for Creutzfeldt–Jakob disease. However, elevated cerebrospinal fluid levels of 14-3-3 can serve as a useful diagnostic aid for for distinguishing Creutzfeldt–Jakob disease from other dementias.
- PRNP methionine/valine (MV) polymorphism
-
The normal polymorphism at codon 129 of the human prion protein (PRNP) gene that codes for the prion protein.
- Protein misfolding cyclic amplification (PMCA)
-
A highly sensitive and specific in vitro prion amplification reaction in which a test sample is mixed with suitable sources of PrPC (usually brain homogenates) and subjected to cycles of sonication and rest; amplified protease-resistant, infectious prions are typically detected by western blot after proteinase K treatment.
- RT-QuIC
-
Real-time quaking-induced conversion (RT-QuIC) is a highly sensitive and specific in vitro test for prion-associated seeding activity in which a test sample is mixed with recombinant PrPC in multiwell plates that are subjected to cycles of shaking and rest. As the reaction progresses, prion-seeded, but apparently non-infectious recombinant PrP amyloid fibrils are detected by enhanced fluorescence of an amyloid-sensitive dye.
Rights and permissions
About this article
Cite this article
Zanusso, G., Monaco, S., Pocchiari, M. et al. Advanced tests for early and accurate diagnosis of Creutzfeldt–Jakob disease. Nat Rev Neurol 12, 325–333 (2016). https://doi.org/10.1038/nrneurol.2016.65
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.65
This article is cited by
-
Seed amplification assay of nasal swab extracts for accurate and non-invasive molecular diagnosis of neurodegenerative diseases
Translational Neurodegeneration (2023)
-
Comparison of cerebrospinal fluid tau, ptau(181), synuclein, and 14-3-3 for the detection of Creutzfeldt–Jakob disease in clinical practice
Journal of Neural Transmission (2022)
-
A single ultrasensitive assay for detection and discrimination of tau aggregates of Alzheimer and Pick diseases
Acta Neuropathologica Communications (2020)
-
Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies
Acta Neuropathologica (2020)
-
Role of prion protein glycosylation in replication of human prions by protein misfolding cyclic amplification
Laboratory Investigation (2019)