Key Points
-
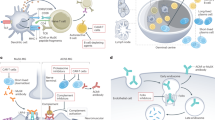
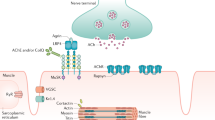
The characteristic muscle weakness in myasthenia gravis (MG) is caused by antibodies directed against the neuromuscular junction
-
MG is divided into subgroups on the basis of specific antibodies, other biomarkers, and clinical characteristics, such as age of onset, presence of thymoma, and involvement of ocular muscles
-
The most common antibodies detected in MG are antibodies against acetylcholine receptors (AChRs), muscle-specific kinase (MuSK) and low-density lipoprotein receptor-related protein 4 (LRP4)
-
Additional antibodies of interest in MG are directed against agrin, titin, KV1.4, ryanodine receptors, collagen Q, and cortactin
-
Therapy should be tailored to the individual patient and guided by MG subgroup, and can include symptomatic drug therapy, immunosuppressive drug therapy, thymectomy and/or supportive therapy
-
The aim of treatment should be normal or near-normal function, which in most patients requires long-term immunosuppressive treatment with a drug combination that is individualized for the patient for optimal effectiveness
Abstract
Myasthenia gravis (MG) is an autoimmune disorder caused by autoantibodies that target the neuromuscular junction, leading to muscle weakness and fatigability. Currently available treatments for the disease include symptomatic pharmacological treatment, immunomodulatory drugs, plasma exchange, thymectomy and supportive therapies. Different autoantibody patterns and clinical manifestations characterize different subgroups of the disease: early-onset MG, late-onset MG, thymoma MG, muscle-specific kinase MG, low-density lipoprotein receptor-related protein 4 MG, seronegative MG, and ocular MG. These subtypes differ in terms of clinical characteristics, disease pathogenesis, prognosis and response to therapies. Patients would, therefore, benefit from treatment that is tailored to their disease subgroup, as well as other possible disease biomarkers, such as antibodies against cytoplasmic muscle proteins. Here, we discuss the different MG subtypes, the sensitivity and specificity of the various antibodies involved in MG for distinguishing between these subtypes, and the value of antibody assays in guiding optimal therapy. An understanding of these elements should be useful in determining how to adapt existing therapies to the requirements of each patient.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gilhus, N. E. Myasthenia and neuromuscular junction. Curr. Opin. Neurol. 25, 523–529 (2012).
Querol, L. & Illa, I. Myasthenia and the neuromuscular junction. Curr. Opin. Neurol. 26, 459–465 (2013).
Verschuuren, J. J.G. M. et al. Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase, and low-density lipoprotein receptor-related protein 4. Autoimmune Rev. 12, 918–923 (2013).
Gilhus, N. E. & Verschuuren, J. J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 14, 1023–1036 (2015).
Heldal, A. T., Owe, J. F., Gilhus, N. E. & Romi, F. Seropositive myasthenia gravis; a nationwide epidemiologic study. Neurology 73, 150–151 (2009).
Carr, A. S. et al. A systematic review of population based epidemiological studies in myasthenia gravis. BMC Neurol. 10, 46 (2010).
Owe, J. F., Daltveit, A. K. & Gilhus, N. E. Causes of death among patients with myasthenia gravis in Norway between 1951 and 2001. J. Neurol. Neurosurg. Psychiatry 77, 203–207 (2006).
Skeie, G. O. et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur. J. Neurol. 17, 893–902 (2010).
Otsuka, K. et al. Collagen Q and anti-MuSK autoantibody competitively suppress agrin/LRP4/MuSK signalling. Sci. Rep. 5, 13928 (2015).
Messeant, J. et al. MuSK frizzled-like domain is critical for mammalian neuromuscular junction formation and maintenance. J. Neurosci. 35, 4926–4941 (2015).
Zisimopoulou, P., Brenner, T., Trakas, N. & Tzartos, S. J. Serological diagnostics in myasthenia gravis based on novel assays and recently identified antigens. Autoimmune Rev. 12, 924–930 (2013).
Romi, F., Skeie, G. O., Gilhus, N. E. & Aarli, J. A. Striational antibodies in myasthenia gravis; reactivity and possible clinical significance. Arch. Neurol. 62, 442–446 (2005).
Romi, F., Aarli, J. A. & Gilhus, N. E. Myasthenia gravis patients with ryanodine receptor antibodies have distinctive clinical features. Eur. J. Neurol. 14, 617–620 (2007).
Suzuki, S. et al. Autoimmune targets of heart and skeletal muscles in myasthenia gravis. Arch. Neurol. 66, 1334–1338 (2009).
Leite, M. I. et al. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain 131, 1940–1952 (2008).
Tsonis, A. I. et al. MuSK autoantibodies in myasthenia gravis detected by cell-based assay: a multinational study. J. Neuroimmunol. 284, 10–17 (2015).
Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 346, 967–989 (2005).
Kordas, G. et al. Direct proof of the in vivo pathogenic role of the AChR autoantibodies from myasthenia gravis patients. PLoS ONE 9, e108327 (2014).
Heldal, A. T., Eide, G. E., Romi, F., Owe, J. F. & Gilhus, N. E. Repeated acetylcholine receptor antibody-concentrations and association to clinical myasthenia gravis development. PLoS ONE 9, e114060 (2014).
Yang, L. et al. Non-radioactive serological diagnosis of myasthenia gravis and clinical features of patients from Tianjin, China. J. Neurol. Sci. 301, 71–76 (2011).
Jacob, S., Viega, S. & Leite, M. I. Presence and pathogenic relevance of antibodies to clustered acetylcholine receptor in ocular and generalized myasthenia gravis. Arch. Neurol. 69, 994–1001 (2012).
Cruz, P. M. R. et al. Clinical features and diagnostic usefulness of antibodies to clustered acetylcholine receptors in the diagnosis of seronegative myasthenia gravis. JAMA Neurol. 72, 642–649 (2015).
Plomp, J. J., Huijbers, M. G., van der Maarel, S. M. & Verschuuren, J. J. Pathogenic IgG4 subclass autoantibodies in MuSK myasthenia gravis. Ann. NY Acad. Sci. 1275, 114–122 (2012).
Kawakami, Y. et al. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology 77, 1819–1828 (2011).
Chang, T. et al. Clinical and serological study of myasthenia gravis using both radioimmunoprecipitation and cell-based assays in a South Asian population. J. Neurol. Sci. 343, 82–87 (2014).
Higuchi, O. et al. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann. Neurol. 69, 418–422 (2011).
Zisimopoulou, P. et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J. Autoimmun. 52, 139–145 (2014).
Pevzner, A. et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibodynegative myasthenia gravis. J. Neurol. 259, 427–435 (2012).
Lu, Y. et al. A role for LRP4 in neuronal cell viability is related to apoE-binding. Brain Res. 1177, 19–28 (2007).
Shen, C. et al. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. J. Clin. Invest. 123, 5190–5202 (2013).
Tzartos, J. S. et al. LRP4 antibodies in serum and CSF from amyotrophic lateral sclerosis patients. Ann. Clin. Trans. Neurol. 2, 80–87 (2014).
Gasperi, C. et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology 82, 1976–1983 (2014).
Zhang, B. et al. Autoantibodies to agrin in myasthenia gravis. PloS ONE 9, e91816 (2014).
Witzemann, V., Chevessier, F., Pacifici, P. G. & Yampolsky, P. The neuromuscular junction: selective remodeling of synaptic regulators at the nerve/muscle interface. Mech. Dev. 130, 402–411 (2013).
Szczudlik, P. et al. Anti-titin antibody in early and late onset myasthenia gravis. Acta Neurol. Scand. 130, 229–233 (2014).
Powers, K. et al. Titin force is enhanced in actively stretched skeletal muscle. J. Exp. Biol. 217, 3629–3636 (2014).
Gautel, M. et al. Titin antibodies in myasthenia gravis: identification of a major antigenic region of titin. Neurol. 43, 1381–1385 (1993).
Romi, F. et al. Anti-voltage-gated potassium channel Kv1.4 antibodies in myasthenia gravis. J. Neurol. 259, 1312–1316 (2012).
Suzuki, S. et al. Cardiac involvements in myasthenia gravis associated with anti-Kv1.4 antibodies. Eur. J. Neurol. 21, 223–230 (2014).
Skeie, G. O. et al. Ryanodine receptor antibodies in myasthenia gravis: epitope mapping and effect on calcium release in vitro. Muscle Nerve 27, 81–89 (2003).
Zoltowska, K. M. et al. Collagen Q: a potential target for autoantibodies in myasthenia gravis. J. Neurol. Sci. 348, 241–244 (2015).
Gallardo, E. et al. Cortactin autoantibodies in myasthenia gravis. Autoimmun. Rev. 13, 1003–1007 (2014).
Gronseth, G. H. & Barohn, R. J. Thymectomy for autoimmune myasthenia gravis (an evidence-based review). Neurology 55, 7–15 (2000).
VanderPluym, J. et al. Clinical characteristics of pediatric myasthenia: a surveillance study. Pediatrics 132, e939–944 (2013).
Guptill, J. T., Sanders, D. B. & Evoli, A. Anti-MuSK antibody myasthenia gravis; clinical findings and response to treatment in two large cohorts. Muscle Nerve 44, 36–40 (2011).
Kerty, E., Elsais, A., Argov, Z., Evoli, A. & Gilhus, N. E. EFNS/ENS guidelines for the treatment of ocular myasthenia gravis. Eur. J. Neurol. 21, 687–693 (2014).
Palace, J., Newsom-Davis, J. & Lecky, B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology 50, 1778–1783 (1998).
Benatar, M., Sanders, D. B., Wolfe, G. I., McDermott, M. P. & Tawil, R. Design of the efficacy of prednisone in the treatment of ocular myasthenia (EPITOME) trial. Ann. NY Acad. Sci. 1275, 17–22 (2012).
Benatar, M. & Kaminski, H. Medical and surgical treatment for ocular myasthenia (review). Cochrane Database Syst. Rev. 12, CD005081 (2012).
Norwood, F. et al. Myasthenia in pregnancy; best practice guidelines from a UK multispeciality working group. J. Neurol. Neurosurg. Psychiatry 85, 538–543 (2014).
Hehir, M. K. et al. Mycophenolate mofetil in AChR-antibody-positive myasthenia gravis; outcomes in 102 patients. Muscle Nerve 41, 593–598 (2010).
The Muscle Study Group. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology 71, 394–399 (2008).
Sanders, D. B. et al. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology 71, 400–406 (2008).
Diaz-Manera, J. et al. Long-lasting treatment of rituximab in MuSK myasthenia. Neurology 78, 189–193 (2012).
Keung, B. et al. Long-term benefit of rituximab in MuSK autoantibody myasthenia gravis patients. J. Neurol. Neurosurg. Psychiatry 84, 1407–1409 (2013).
Iorio, R. et al. Efficacy and safety of rituximab for myasthenia gravis: a systematic review and meta-analysis. J. Neurol. 262, 1115–1119 (2015).
Mandawat, A. et al. Comparative analysis of therapeutic options used for myasthenia gravis. Ann. Neurol. 68, 797–805 (2010).
Barth, D. et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 76, 2017–2023 (2011).
Gilhus, N. E. Acute treatment for myasthenia gravis. Nat. Rev. Neurol. 7, 132–134 (2011).
Gajdos, P., Chevret, S. & Toyka, K. V. Intravenous immunoglobulin for myasthenia gravis (review). Cochrane Database Syst. Rev. 12, CD002277 (2012).
Lazaridis, K. et al. Specific adsorbents for myasthenia gravis autoantibodies using mutants of the muscle nicotinic acetylcholine receptor extracellular domains. J. Neuroimmunol. 278, 19–25 (2015).
Steinman, L. The road not taken: antigen-specific therapy and neuroinflammatory disease. JAMA Neurol. 70, 1100–1101 (2013).
Marx, A. et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun. Rev. 12, 875–884 (2013).
Cea, G., Benatar, M., Verdugo, R. J. & Salinas, R. A. Thymectomy for non-thymomatous myasthenia gravis (review). Cochrane Database Syst. Rev. 10, CD008111 (2013).
Ye, B. et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J. Surg. Oncol. 11, 157–162 (2013).
Gilhus, N. E., Nacu, A., Andersen, J. B. & Owe, J. F. Myasthenia gravis and risks for comorbidity. Eur. J. Neurol. 22, 17–23 (2015).
Chiou-Tan, F. Y. & Gilchrist, J. M. Repetitive nerve stimulation and single-fiber electromyography in the evaluation of patients with suspected myasthenia gravis or Lambert–Eaton myasthenic syndrome: review of recent literature. Muscle Nerve 52, 455–462 (2015).
Huijbers, M. G. et al. Pathogenic immune mechanisms at the neuromuscular synapse: the role of specific antibody-binding epitopes in myasthenia gravis. J. Intern. Med. 275, 12–26 (2013).
Stiegler, A. L., Burden, S. J. & Hubbard, S. R. Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK. J. Mol. Biol. 364, 424–433 (2006).
Till, J. H. et al. Crystal structure of the MuSK tyrosine kinase: insights into receptor autoregulation. Structure 10, 1187–1196 (2002).
Koneczny, I., Cossins, J., Waters, P., Beeson, D. & Vincent, A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS ONE 8, e80695 (2013).
Stiegler, A. L., Burden, S. J. & Hubbard, S. R. Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK. J. Mol. Biol. 393, 1–9 (2009).
Choi, H. Y. et al. Lrp4, a novel receptor for dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS ONE 4, e7930 (2009).
Zong, Y. et al. Structural basis of agrin–LRP4–MuSK signaling. Genes Dev. 26, 247–258 (2012).
Zhang, B. et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch. Neurol. 69, 445–451 (2012).
Acknowledgements
N.E.G., G.O.S. and F.R. have received funding from Torbjørg Hauge's Legacy for Neurological Research and from the Norwegian Muscle Disease Association. S.J.T., K.L. and P.Z. have received grants from the Muscular Dystrophy Association of the USA and from the Greek National Strategic Reference Framework (NeuroID ISR-3257).
Author information
Authors and Affiliations
Contributions
All authors researched data for the Review, made substantial contributions to the discussion of the content of the article and reviewed and edited the manuscript before submission. N.E.G and S.T. wrote the article.
Corresponding author
Ethics declarations
Competing interests
N.E.G. has received speaker's honoraria from Octapharma, Baxter and Merck Serono. P.Z. is co-inventor in a patent related to myasthenia gravis therapy and diagnosis. S.T. is co-inventor in two patnets related to myasthenia gravis therapy and diagnosis, and is shareholder and scientific advisor of Tzartos Neurodiagnostics. Other authors declare no competing interests.
PowerPoint slides
Glossary
- Antigenic modulation
-
Bivalent antibodies can cause crosslinking of receptors and subsequent receptor internalization.
- Epitope pattern
-
An epitope is a localized region on an antigen capable of eliciting an immune response, and the epitope pattern refers to all epitopes involved in an immune response.
- MG crisis
-
Severe worsening of myasthenic weakness that requires intubation or noninvasive ventilation to avoid intubation.
Rights and permissions
About this article
Cite this article
Gilhus, N., Skeie, G., Romi, F. et al. Myasthenia gravis — autoantibody characteristics and their implications for therapy. Nat Rev Neurol 12, 259–268 (2016). https://doi.org/10.1038/nrneurol.2016.44
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.44
This article is cited by
-
Mendelian randomization analyses of known and suspected risk factors and biomarkers for myasthenia gravis overall and by subtypes
BMC Neurology (2024)
-
Mendelian randomization study revealed a gut microbiota-neuromuscular junction axis in myasthenia gravis
Scientific Reports (2024)
-
Ocular Myasthenia Gravis
Current Treatment Options in Neurology (2023)
-
Oculomotor fatigability with decrements of saccade and smooth pursuit for diagnosis of myasthenia gravis
Journal of Neurology (2023)
-
miRNAs as the important regulators of myasthenia gravis: involvement of major cytokines and immune cells
Immunologic Research (2023)