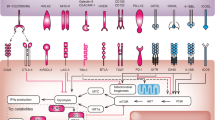
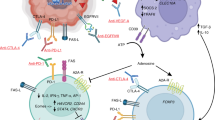
Key Points
-
The prognosis for glioblastoma patients is poor, with median overall survival of approximately 15–17 months
-
Immunotherapy has clinical benefits in other advanced tumours, such as melanoma and lung cancer, for which conventional therapies have had limited success
-
The blood–brain barrier prevents macromolecules from entering the CNS, but readily allows traffic of activated lymphocytes; thus, communication occurs between the CNS and the immune system
-
The success of immunotherapy in other cancers, and the current understanding of the interaction between the brain and the immune system provide a rationale for exploration of immune checkpoint inhibitors in glioblastoma
-
Tumour progression could involve multiple immunosuppressive mechanisms, making combination of immunotherapeutic agents that target different pathways a promising approach
-
Clinical trials evaluating immune checkpoint inhibitors in glioblastoma patients are ongoing
Abstract
Glioblastoma is the most common primary brain tumour in adults. Prognosis is poor: even with the current gold-standard first-line treatment—maximal safe resection and combination of radiotherapy with temozolomide chemotherapy—the median overall survival time is only approximately 15–17 months, because the tumour recurs in virtually all patients, and no commonly accepted standard treatment for recurrent disease exists. Several targeted agents have failed to improve patient outcomes in glioblastoma. Immunotherapy with immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab has provided relevant clinical improvements in other advanced tumours for which conventional therapies have had limited success, making immunotherapy an appealing strategy in glioblastoma. This Review summarizes current knowledge on immune checkpoint modulators and evaluates their potential role in glioblastoma on the basis of preclinical studies and emerging clinical data. Furthermore, we discuss challenges that need to be considered in the clinical development of drugs that target immune checkpoint pathways in glioblastoma, such as specific properties of the immune system in the CNS, issues with radiological response assessment, and potential interactions with established and emerging treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16 (Suppl. 4), iv1–iv63 (2014).
Gilbert, M. R. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 699–708 (2014).
Temodar (temozolomide) [prescribing information]. http://www.merck.com/product/usa/pi_circulars/t/temodar_capsules/temodar_pi.pdf (Merck & Co., Inc. 2014).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Omuro, A. M., Faivre, S. & Raymond, E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol. Cancer Ther. 6, 1909–1919 (2007).
Woehrer, A., Bauchet, L. & Barnholtz-Sloan, J. S. Glioblastoma survival: has it improved? Evidence from population-based studies. Curr. Opin. Neurol. 27, 666–674 (2014).
Weller, M. et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 15, e395–e403 (2014).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
O'Day, S. J. et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann. Oncol. 21, 1712–1717 (2010).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Motzer, R. J. et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 33, 1430–1437 (2015).
Rizvi, N. A. et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 16, 257–265 (2015).
Opdivo (nivolumab) [prescribing information]. http://www.opdivo.bmscustomerconnect.com/gateway, (Bristol-Myers Squibb Company, 2014).
Keytruda (pembrolizumab) [prescribing information]. http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf, (Merck & Co., Inc. 2015).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 39, 1–10 (2013).
Driessens, G., Kline, J. & Gajewski, T. F. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol. Rev. 229, 126–144 (2009).
Jackson, C., Ruzevick, J., Phallen, J., Belcaid, Z. & Lim, M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin. Dev. Immunol. 2011, 1–21 (2011).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 12, 252–264 (2012).
Bakdash, G., Sittig, S. P., van Dijk, T., Figdor, C. G. & de Vries, I. J. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front. Immunol. 4, 53 (2013).
Joller, N. et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 186, 1338–1342 (2011).
Hastings, W. D. et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 39, 2492–2501 (2009).
Tarhini, A. A. & Iqbal, F. CTLA-4 blockade: therapeutic potential in cancer treatments. Onco Targets Ther. 3, 15–25 (2010).
Margolin, K. et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 13, 459–465 (2012).
Queirolo, P. et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J. Neurooncol. 118, 109–116 (2014).
Yervoy (ipilimumab) [prescribing information]. http://www.yervoy.com, (Bristol-Myers Squibb Company, 2013).
Di Giacomo, A. M. et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 13, 879–886 (2012).
Luke, J. J. & Ott, P. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget. 6, 3479–3492 (2015).
Weber, J. S. et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 31, 4311–4318 (2013).
Robert, C. et al. Anti-programmed-death- receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 384, 1109–1117 (2014).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Antonia, S. J. et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 32 (15 Suppl.), 8113 (2014).
Antonia, S. J. et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J. Clin. Oncol. 32 (15 Suppl.), 8023 (2014).
Gettinger, S. N. et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: safety, efficacy, and correlation of outcomes with PD-L1 status. J. Clin. Oncol. 32 (15 Suppl.), 8024 (2014).
Rizvi, N. A. et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J. Clin. Oncol. 32 (15 Suppl.), 8022 (2014).
Garon, E. B. et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J. Clin. Oncol. 32 (15 Suppl.), 8020 (2014).
Hafler, D. A. & Kuchroo, V. TIMs: central regulators of immune responses. J. Exp. Med. 205, 2699–2701 (2008).
Anderson, A. C. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2, 393–398 (2014).
Sierro, S., Romero, P. & Speiser, D. E. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin. Ther. Targets. 15, 91–101 (2011).
Woo, S. R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012).
Downey, S. G. et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin. Cancer Res. 13, 6681–6688 (2007).
Juszczak, A., Gupta, A., Karavitaki, N., Middleton, M. R. & Grossman, A. B. Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur. J. Endocrinol. 167, 1–5 (2012).
Weber, J. S. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am. Soc. Clin. Oncol. Educ. Book 174–177 (2012).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Dranoff, G. Immunotherapy at large: balancing tumor immunity and inflammatory pathology. Nat. Med. 19, 1100–1101 (2013).
Fecher, L. A., Agarwala, S. S., Hodi, F. S. & Weber, J. S. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 18, 733–743 (2013).
Heimberger, A. B. & Sampson, J. H. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 13, 3–13 (2011).
Raizer, J. Issues in developing drugs for primary brain tumors: barriers and toxicities. Toxicol. Pathol. 39, 152–157 (2011).
Taube, J. M. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20, 5064–5074 (2014).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Vauleon, E., Avril, T., Collet, B., Mosser, J. & Quillien, V. Overview of cellular immunotherapy for patients with glioblastoma. Clin. Dev. Immunol. 2010 (2010).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature http://dx.doi.org/10.1038/nature14432,.
Reardon, D. A. et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 16, 1441–1458 (2014).
Muller, C. et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 6, 247ra101 (2014).
Preusser, M. Neuro-oncology: a step towards clinical blood biomarkers of glioblastoma. Nat. Rev. Neurol. 10, 681–682 (2014).
Tran, T. T. et al. Inhibiting TGF-β signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 9, 259–270 (2007).
Wainwright, D. A. et al. Durable therapeutic efficacy utilizing combinatorial blockade against, IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 20, 5290–5301 (2014).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84–88 (2007).
Fecci, P. E. et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 66, 3294–3302 (2006).
Ooi, Y. C. et al. The role of regulatory T-cells in glioma immunology. Clin. Neurol. Neurosurg. 119, 125–132 (2014).
Mirghorbani, M., Van Gool, S. & Rezaei, N. Myeloid-derived suppressor cells in glioma. Expert Rev. Neurother. 13, 1395–1406 (2013).
Vlahovic, G., Fecci, P. E., Reardon, D. & Sampson, J. H. Programmed death-ligand 1(PD-L1) as an immunotherapy target in patients with glioblastoma. Neuro Oncol. 17, 1043–1045 (2015).
Berghoff, A. S. et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 17, 1064–1075 (2014).
Bloch, O. et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 19, 3165–3175 (2013).
Velcheti, V. et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Invest. 94, 107–116 (2014).
Doucette, T. et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol. Res. 1, 112–122 (2013).
Bhat, K. P. et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24, 331–346 (2013).
Avril, T. et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2, 3-dioxygenase (IDO) on tumour-specific T cell functions. J. Neuroimmunol. 225, 22–33 (2010).
Wintterle, S. et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 63, 7462–7467 (2003).
Magnus, T. et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J. Neurosci. 25, 2537–2546 (2005).
Berghoff, A. S. et al. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin. Neuropathol. 33, 42–49 (2014).
Berghoff, A. S. et al. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 66, 289–299 (2015).
Berghoff, A. S. et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunol (in press). (2015).
Han, S. et al. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. Br. J. Cancer 110, 2560–2568 (2014).
Yue, Q. et al. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J. Neurooncol. 116, 251–259 (2014).
Rutledge, W. C. et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin. Cancer Res. 19, 4951–4960 (2013).
Kjaergaard, J. et al. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 60, 5514–5521 (2000).
Fecci, P. E. et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 13, 2158–2167 (2007).
Reardon, D. A. et al. Immune checkpoint blockade for glioblastoma: Preclinical activity of single agent and combinatorial therapy. J. Clin. Oncol. 32 (15 Suppl.), 2084 (2014).
Zeng, J. et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 86, 343–349 (2013).
Belcaid, Z. et al. Focal radiation therapy combined with 4–1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE 9, e101764 (2014).
Schumacher, T. et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 512, 324–327 (2014).
Schuster, J. et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 17, 854–861 (2015).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Chaichana, K. L., Pinheiro, L. & Brem, H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther. Deliv. 6, 353–369 (2015).
Sims, J. S., Ung, T. H., Neira, J. A., Canoll, P. & Bruce, J. N. Biomarkers for glioma immunotherapy: the next generation. J. Neurooncol. 3, 359–372 (2015).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Ribas, A., Chmielowski, B. & Glaspy, J. A. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin. Cancer Res. 15, 7116–7118 (2009).
Wolchok, J. How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma. Ann. Oncol. 23, (Suppl. 8) viii15–viii21 (2012).
Nishino, M. et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin. Cancer Res. 19, 3936–3943 (2013).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972 (2010).
Papadatos-Pastos, D., Soultati, A. & Harries, M. Targeting brain metastases in patients with melanoma. Biomed. Res. Int. 2013, 186563 (2013).
Park, B., Yee, C. & Lee, K. M. The effect of radiation on the immune response to cancers. Int. J. Mol. Sci. 15, 927–943 (2014).
Frey, B. et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation – implications for cancer therapies. Curr. Med. Chem. 19, 1751–1764 (2012).
Grossman, S. A. et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 17, 5473–5480 (2011).
Grimaldi, A. M. et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 3, e28780 (2014).
Nicholas, S. et al. Current trends in glioblastoma multiforme treatment: radiation therapy and immune checkpoint inhibitors. Brain Tumor Res. Treat. 1, 2–8 (2013).
Stupp, R. et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 2712–2718 (2010).
Zitvogel, L. et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118, 1991–2001 (2008).
Sampson, J. H. et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 13, 324–333 (2011).
Sanchez-Perez, L. A. et al. Myeloablative temozolomide enhances CD8(+) T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS ONE 8, e59082 (2013).
Dudley, M. E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002).
Avastin (bevacizumab) [prescribing information]. http://www.gene.com/download/pdf/avastin_prescribing.pdf, (Genentech, Inc. 2014).
Mansfield, A. S., Nevala, W. K., Lieser, E. A., Leontovich, A. A. & Markovic, S. N. The immunomodulatory effects of bevacizumab on systemic immunity in patients with metastatic melanoma. Oncoimmunology 2, e24436 (2013).
Terme, M. et al. Modulation of immunity by antiangiogenic molecules in cancer. Clin. Dev. Immunol. 2012, 8 (2012).
Nishino, M., Giobbie-Hurder, A., Ramaiya, N. H. & Hodi, F. S. Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J. Immunother. Cancer 2, 40 (2014).
Schaff, L. R. et al. Ipilimumab for recurrent glioblastoma (GBM). J. Clin. Oncol. 32 (15 Suppl.). 32, e13026 (2014).
Chinot, O. L. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 709–722 (2014).
Kaal, E. C. & Vecht, C. J. The management of brain edema in brain tumors. Curr. Opin. Oncol. 16, 593–600 (2004).
Hempen, C., Weiss, E. & Hess, C. F. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Support Care Cancer 10, 322–328 (2002).
Min, L., Vaidya, A. & Becker, C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur. J. Endocrinol. 164, 303–307 (2011).
Acknowledgements
M. Daniels from inScience Communications in Philadelphia, PA, USA, provided editorial support for writing this article, comprising compilation of tables, management of the reference database, and editing of the manuscript text before submission. The editorial support was funded by Bristol-Myers Squibb. D.A.H. has received the National Multiple Sclerosis Society Collaborative Research Centre Award CA1061-A-18, NIH grants P01 AI045757, U19 AI046130, U19 AI070352, and P01 AI039671, and research support from the Nancy Taylor Foundation for Chronic Diseases and the Penates Foundation. J.H.S. has received the NIH grants R01-CA177476-02, R01-NS086943-01, P50-CA190991-01, and 2R25-NS065731-06, and research support from the Accelerate Brain Cancer Cure and Pediatric Brain Tumor Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, substantial contribution to discussing of content, writing, editing and reviewing of the article.
Corresponding author
Ethics declarations
Competing interests
M.P. has received honoraria, research support (unrestricted grants), and travel support (to scientific meetings) from Bristol-Myers Squibb, Böhringer-Ingelheim, GlaxoSmithKline, Mundipharma and Roche. M.L. has received research support from Agenus, Arbor Pharmaceuticals, Bristol-Myers Squibb, Celldex Therapeutics, and ImmunoCellular Therapeutics, and has served as a consultant for Bristol-Myers Squibb. D.A.H. has consulted for Allergan Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Genzyme Sanofi-Aventis, MedImmune, Mylan Pharmaceuticals, Novartis Pharmaceuticals, Questcor and Teva Neuroscience, and has received grant support from Bristol-Myers Squibb. D.A.R. has received financial compensation for participation in advisory boards for Amgen, Cavion, Genentech/Roche, Midatech Pharma, Momenta Pharmaceuticals, Novartis and Stemline Therapeutics, served as a member of speakers' bureaus for Genentech/Roche and Merck, and received research support from Celldex Therapeutics and Incyte. J.H.S. declares no competing interests.
Rights and permissions
About this article
Cite this article
Preusser, M., Lim, M., Hafler, D. et al. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol 11, 504–514 (2015). https://doi.org/10.1038/nrneurol.2015.139
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.139
This article is cited by
-
Global stability and parameter analysis reinforce therapeutic targets of PD-L1-PD-1 and MDSCs for glioblastoma
Journal of Mathematical Biology (2024)
-
Comprehensive analysis of the clinical and biological significances of cholesterol metabolism in lower-grade gliomas
BMC Cancer (2023)
-
Recent advances and future challenges of tumor vaccination therapy for recurrent glioblastoma
Cell Communication and Signaling (2023)
-
Nucleic acid drug vectors for diagnosis and treatment of brain diseases
Signal Transduction and Targeted Therapy (2023)
-
KDM6B-mediated reprogramming of myeloid cells regulates the response to immunotherapy
Nature Cancer (2023)