Abstract
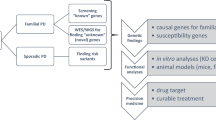
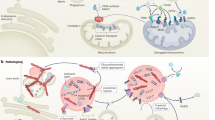
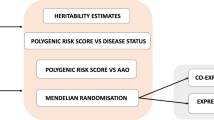
Parkinson disease (PD) is a multifactorial neurodegenerative disease that was long considered the result of environmental factors. In the past 15 years, however, a genetic aetiology for PD has begun to emerge. Here, we review results from linkage and next-generation sequencing studies of familial parkinsonism, as well as candidate gene and genome-wide association findings in sporadic PD. In these studies, many of the genetic findings overlap, despite different designs and study populations, highlighting novel therapeutic targets. The molecular results delineate a sequence of pathological events whereby deficits in synaptic exocytosis and endocytosis, endosomal trafficking, lysosome-mediated autophagy and mitochondrial maintenance increase susceptibility to PD. These discoveries provide the rationale, molecular insight and research tools to develop neuroprotective and disease-modifying therapies.
Key Points
-
Parkinson disease (PD) is a multifactorial disorder that, in most cases, results from genetic and environmental factors
-
Approaches to study the genetics of PD include linkage analysis, genome sequencing and association studies
-
Many genes, mutations and polymorphisms have implicated in PD pathogenesis
-
Genetic discoveries highlight biological processes and pathways that are consistently perturbed in idiopathic PD, although individual results are seldom useful for a patient diagnosis
-
Identification of such genetic variability may inform the design of clinical trials and future therapeutic strategies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson's disease. Lancet Neurol. 5, 525–535 (2006).
Schrag, A. & Schott, J. M. Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. Lancet Neurol. 5, 355–363 (2006).
Lang, A. E. & Lozano, A. M. Parkinson's disease. Second of two parts. N. Engl. J. Med. 339, 1130–1143 (1998).
Lang, A. E. & Lozano, A. M. Parkinson's disease. First of two parts. N. Engl. J. Med. 339, 1044–1053 (1998).
Fahn, S. Description of Parkinson's disease as a clinical syndrome. Ann. N. Y. Acad. Sci. 991, 1–14 (2003).
Langston, J. W. The Parkinson's complex: parkinsonism is just the tip of the iceberg. Ann. Neurol. 59, 591–596 (2006).
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M. & Morris, J. G. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844 (2008).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 27, 349–356 (2012).
Fahn, S. The spectrum of levodopa-induced dyskinesias. Ann. Neurol. 47, S2–S9 (2000).
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Goedert, M., Spillantini, M. G., Del Tredici, K. & Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 (2013).
Halliday, G. M., Holton, J. L., Revesz, T. & Dickson, D. W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 122, 187–204 (2011).
Farrer, M. J. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet. 7, 306–318 (2006).
Casals, J., Elizan, T. S. & Yahr, M. D. Postencephalitic parkinsonism—a review. J. Neural Transm. 105, 645–676 (1998).
Langston, J. W., Ballard, P., Tetrud, J. W. & Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983).
Tanner, C. M. et al. Parkinson disease in twins: an etiologic study. JAMA 281, 341–346 (1999).
Wirdefeldt, K., Gatz, M., Reynolds, C. A., Prescott, C. A. & Pedersen, N. L. Heritability of Parkinson disease in Swedish twins: a longitudinal study. Neurobiol. Aging 32, 1923.e1–1923.e8 (2011).
Polymeropoulos, M. H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Kruger, R. et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108 (1998).
Zarranz, J. J. et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 (2004).
Proukakis, C. et al. A novel α-synuclein missense mutation in Parkinson disease. Neurology 80, 1062–1064 (2013).
Appel-Cresswell, S. et al. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson disease. Mov. Disord. http://dx.doi.org/10.1002/mds.25421.
Lesage, S. et al. G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 73, 459–471 (2013).
Singleton, A. B. et al. α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 (2003).
Chartier-Harlin, M. C. et al. α-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 (2004).
Ibáñez, P. et al. Causal relation between α-synuclein gene duplication and familial Parkinson's disease. Lancet 364, 1169–1171 (2004).
Fuchs, J. et al. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology 68, 916–922 (2007).
Nishioka, K. et al. Expanding the clinical phenotype of SNCA duplication carriers. Mov. Disord. 24, 1811–1819 (2009).
Ross, O. A. et al. Genomic investigation of α-synuclein multiplication and parkinsonism. Ann. Neurol. 63, 743–750 (2008).
Farrer, M. et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 50, 293–300 (2001).
Pals, P. et al. α-Synuclein promoter confers susceptibility to Parkinson's disease. Ann. Neurol. 56, 591–595 (2004).
Winkler, S. et al. α-Synuclein and Parkinson disease susceptibility. Neurology 69, 1745–1750 (2007).
Rajput, A. et al. α-synuclein polymorphisms are associated with Parkinson's disease in a Saskatchewan population. Mov. Disord. 24, 2411–2414 (2009).
Pankratz, N. et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum. Genet. 124, 593–605 (2009).
Maraganore, D. M. et al. Collaborative analysis of α-synuclein gene promoter variability and Parkinson disease. JAMA 296, 661–670 (2006).
Chiba-Falek, O., Touchman, J. W. & Nussbaum, R. L. Functional analysis of intra-allelic variation at NACP-Rep1 in the α-synuclein gene. Hum. Genet. 113, 426–431 (2003).
Chiba-Falek, O., Kowalak, J. A., Smulson, M. E. & Nussbaum, R. L. Regulation of α-synuclein expression by poly (ADP ribose) polymerase-1 (PARP-1) binding to the NACP-Rep1 polymorphic site upstream of the SNCA gene. Am. J. Hum. Genet. 76, 478–492 (2005).
Cronin, K. D. et al. Expansion of the Parkinson disease-associated SNCA-Rep1 allele upregulates human α-synuclein in transgenic mouse brain. Hum. Mol. Genet. 18, 3274–3285 (2009).
Nishioka, K. et al. Association of α-, β-, and γ-synuclein with diffuse Lewy body disease. Arch. Neurol. 67, 970–975 (2010).
Scholz, S. W. et al. SNCA variants are associated with increased risk for multiple system atrophy. Ann. Neurol. 65, 610–614 (2009).
Cookson, M. R. Cellular effects of LRRK2 mutations. Biochem. Soc. Trans. 40, 1070–1073 (2012).
Zimprich, A. et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 (2004).
Paisán-Ruíz, C. et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595–600 (2004).
Kachergus, J. et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet. 76, 672–680 (2005).
Aasly, J. O. et al. Novel pathogenic LRRK2 p.Asn1437His substitution in familial Parkinson's disease. Mov. Disord. 25, 2156–2163 (2010).
Ozelius, L. J. et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 354, 2424–2425 (2006).
Lesage, S. et al. G2019S LRRK2 mutation in French and North African families with Parkinson's disease. Ann. Neurol. 58, 2784–2787 (2005).
Ross, O. A. et al. Lrrk2 and Lewy body disease. Ann. Neurol. 59, 388–393 (2006).
Ujiie, S. et al. LRRK2 I2020T mutation is associated with tau pathology. Parkinsonism Relat. Disord. 18, 2819–2823 (2012).
Satake, W. et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 41, 1303–1307 (2009).
Do, C. B. et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 7, e1002141 (2011).
Mata, I. F. et al. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics 6, 171–177 (2005).
Rubio, J. P. et al. Deep sequencing of the LRRK2 gene in 14,002 individuals reveals evidence of purifying selection and independent origin of the p.Arg1628Pro mutation in Europe. Hum. Mutat. 33, 1087–1098 (2012).
Tan, E. K. et al. Multiple LRRK2 variants modulate risk of Parkinson disease: a Chinese multicenter study. Hum. Mutat. 31, 561–568 (2010).
Ross, O. A. et al. Association of LRRK2 exonic variants with susceptibility to Parkinson's disease: a case–control study. Lancet Neurol. 10, 898–908 (2011).
Farrer, M. J. et al. Lrrk2 G2385R is an ancestral risk factor for Parkinson's disease in Asia. Parkinsonism Relat. Disord. 13, 2389–2392 (2007).
Vandrovcova, J. et al. Disentangling the role of the tau gene locus in sporadic tauopathies. Curr. Alzheimer Res. 7, 726–734 (2010).
Pankratz, N. et al. Meta-analysis of Parkinson's disease: identification of a novel locus, RIT2. Ann. Neurol. 71, 370–384 (2012).
Skipper, L. et al. Linkage disequilibrium and association of MAPT H1 in Parkinson disease. Am. J. Hum. Genet. 75, 669–677 (2004).
Zody, M. C. et al. Evolutionary toggling of the MAPT 17q21.31 inversion region. Nat. Genet. 40, 1076–1083 (2008).
Baker, M. et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8, 711–715 (1999).
Pittman, A. M. et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J. Med. Genet. 42, 837–846 (2005).
Sundar, P. D. et al. Two sites in the MAPT region confer genetic risk for Guam ALS/PDC and dementia. Hum. Mol. Genet. 16, 295–306 (2007).
Hoglinger, G. U. et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699–705 (2011).
Williams-Gray, C. H. et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 132, 2958–2969 (2009).
Naj, A. C. et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat. Genet. 43, 436–441 (2011).
Hollingworth, P. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 43, 429–435 (2011).
Bekpen, C., Tastekin, I., Siswara, P., Akdis, C. A. & Eichler, E. E. Primate segmental duplication creates novel promoters for the LRRC37 gene family within the 17q21.31 inversion polymorphism region. Genome Res. 22, 1050–1058 (2012).
Chartier-Harlin, M. C. et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am. J. Hum. Genet. 89, 398–406 (2011).
Schulte, E. C. et al. Variants in eukaryotic translation initiation factor 4G1 in sporadic Parkinson's disease. Neurogenetics 13, 281–285 (2012).
Nuytemans, K. et al. Whole exome sequencing of rare variants in EIF4G1 and VPS35 in Parkinson disease. Neurology 80, 982–989 (2013).
Narendra, D., Walker, J. E. & Youle, R. Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harbor Perspect. Biol. 4, a011338 (2012).
Kahle, P. J., Waak, J. & Gasser, T. DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders. Free Radic. Biol. Med. 47, 1354–1361 (2009).
Fallon, L. et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat. Cell Biol. 8, 834–842 (2006).
Abbas, N. et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. Hum. Mol. Genet. 8, 567–574 (1999).
van de Warrenburg, B. P. et al. Clinical and pathologic abnormalities in a family with parkinsonism and parkin gene mutations. Neurology 56, 555–557 (2001).
Samaranch, L. et al. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain 133, 1128–1142 (2010).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Valente, E. M. et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 (2004).
Ishihara-Paul, L. et al. PINK1 mutations and parkinsonism. Neurology 71, 896–902 (2008).
Bonifati, V. et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 (2003).
Annesi, G. et al. DJ-1 mutations and parkinsonism–dementia–amyotrophic lateral sclerosis complex. Ann. Neurol. 58, 803–807 (2005).
Attar, N. & Cullen, P. J. The retromer complex. Adv. Enzyme Regul. 50, 216–236 (2010).
McGough, I. J. & Cullen, P. J. Recent advances in retromer biology. Traffic 12, 963–971 (2011).
Vilarino-Guell, C. et al. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89, 162–167 (2011).
Zimprich, A. et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89, 168–175 (2011).
Lesage, S. et al. Identification of VPS35 mutations replicated in French families with Parkinson disease. Neurology 78, 1449–1450 (2012).
Sharma, M. et al. A multi-centre clinico-genetic analysis of the VPS35 gene in Parkinson disease indicates reduced penetrance for disease-associated variants. J. Med. Genet. 49, 721–726 (2012).
Ando, M. et al. VPS35 mutation in Japanese patients with typical Parkinson's disease. Mov. Disord. 27, 1413–1417 (2012).
Kumar, K. R. et al. Frequency of the D620N mutation in VPS35 in Parkinson disease. Arch. Neurol. 69, 1360–1364 (2012).
Edvardson, S. et al. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS ONE 7, e36458 (2012).
Köroğlu, C., Baysal, L., Cetinkaya, M., Karasoy, H. & Tolun, A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat. Disord. 19, 320–324 (2012).
Vauthier, V. et al. Homozygous deletion of an 80 kb region comprising part of DNAJC6 and LEPR genes on chromosome 1P31.3 is associated with early onset obesity, mental retardation and epilepsy. Mol. Genet. Metab. 106, 345–350 (2012).
Diaz, A. et al. Gaucher disease: the N370S mutation in Ashkenazi Jewish and Spanish patients has a common origin and arose several thousand years ago. Am. J. Hum. Genet. 64, 1233–1238 (1999).
Tsuji, S. et al. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher's disease. N. Engl. J. Med. 316, 570–575 (1987).
Horowitz, M. et al. Prevalence of glucocerebrosidase mutations in the Israeli Ashkenazi Jewish population. Hum. Mutat. 12, 240–244 (1998).
Sidransky, E. & Lopez, G. The link between the GBA gene and parkinsonism. Lancet Neurol. 11, 986–998 (2012).
Aharon-Peretz, J., Rosenbaum, H. & Gershoni-Baruch, R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 351, 1972–1977 (2004).
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Farrer, M. J. et al. Glucosidase-beta variations and Lewy body disorders. Parkinsonism Relat. Disord. 15, 414–416 (2009).
Tsuang, D. et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 79, 1944–1950 (2012).
Sardi, S. P., Singh, P., Cheng, S. H., Shihabuddin, L. S. & Schlossmacher, M. G. Mutant GBA1 expression and synucleinopathy risk: first insights from cellular and mouse models. Neurodegener. Dis. 10, 195–202 (2012).
Tansey, M. G. & Goldberg, M. S. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 37, 510–518 (2010).
Ahmed, I. et al. Association between Parkinson's disease and the HLA-DRB1 locus. Mov. Disord. 27, 1104–1110 (2012).
Hamza, T. H. et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat. Genet. 42, 781–785 (2010).
Simón-Sánchez, J. et al. Genome-wide association study confirms extant PD risk loci among the Dutch. Eur. J. Hum. Genet. 19, 655–661 (2011).
UK Parkinson's Disease Consortium & Wellcome Trust Case Control Consortium. Dissection of the genetics of Parkinson's disease identifies an additional association 5′ of SNCA and multiple associated haplotypes at 17q21. Hum. Mol. Genet. 20, 345–353 (2011).
Lee, D. W., Wu, X., Eisenberg, E. & Greene, L. E. Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J. Cell Sci. 119, 3502–3512 (2006).
Macleod, D. A. et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron 77, 425–439 (2013).
Hill-Burns, E. M. et al. A genetic basis for the variable effect of smoking/nicotine on Parkinson's disease. Pharmacogenomics J. http://dx.doi.org/10.1038/tpj.2012.38.
Hamza, T. H. et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson's disease modifier gene via interaction with coffee. PLoS Genet. 7, e1002237 (2011).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Farrer, M. J. et al. DCTN1 mutations in Perry syndrome. Nat. Genet. 41, 163–165 (2009).
Puls, I. et al. Mutant dynactin in motor neuron disease. Nat. Genet. 33, 455–456 (2003).
Ramirez, A. et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 38, 1184–1191 (2006).
Di Fonzo, A. et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology 68, 1557–1562 (2007).
Morgan, N. V. et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat. Genet. 38, 752–754 (2006).
Paisán-Ruíz, C. et al. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann. Neurol. 65, 19–23 (2009).
Shojaee, S. et al. Genome-wide linkage analysis of a Parkinsonian-pyramidal syndrome pedigree by 500 K SNP arrays. Am. J. Hum. Genet. 82, 1375–1384 (2008).
Di Fonzo, A. et al. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology 72, 240–245 (2009).
Korvatska, O. et al. Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum. Mol. Genet. http://dx.doi.org/10.1093/hmg/ddt180.
Simon, D. K., Lin, M. T. & Pascual-Leone, A. “Nature versus nurture” and incompletely penetrant mutations. J. Neurol. Neurosurg. Psychiatry 72, 686–689 (2002).
Dickson, D. et al. Pathology of PD in monozygotic twins with a 20-year discordance interval. Neurology 56, 981–982 (2001).
Piccini, P., Burn, D. J., Ceravolo, R., Maraganore, D. & Brooks, D. J. The role of inheritance in sporadic Parkinson's disease: evidence from a longitudinal study of dopaminergic function in twins. Ann. Neurol. 45, 577–582 (1999).
Goldstein, D. B. Common genetic variation and human traits. N. Engl. J. Med. 360, 1696–1698 (2009).
Ginsburg, G. S. & Willard, H. F. Genomic and personalized medicine: foundations and applications. Transl. Res. 154, 277–287 (2009).
Farrer, M. et al. Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann. Neurol. 55, 174–179 (2004).
Farrer, M. et al. α-Synuclein gene haplotypes are associated with Parkinson's disease. Hum. Mol. Genet. 10, 1847–1851 (2001).
Brooks, J. et al. Parkin and PINK1 mutations in early-onset Parkinson's disease: comprehensive screening in publicly available cases and control. J. Med. Genet. 46, 375–381 (2009).
Nalls, M. A. et al. Measures of autozygosity in decline: globalization, urbanization, and its implications for medical genetics. PLoS Genet. 5, e1000415 (2009).
Simón-Sánchez, J. et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 41, 1308–1312 (2009).
Mollenhauer, B., El-Agnaf, O. M., Marcus, K., Trenkwalder, C. & Schlossmacher, M. G. Quantification of α-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark. Med. 4, 683–699 (2010).
Burre, J. et al. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667 (2010).
Cooper, A. A. et al. α-Synuclein blocks ER–Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313, 324–328 (2006).
Masliah, E. et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 (2000).
Lewis, J. et al. In vivo silencing of α -synuclein using naked siRNA. Mol. Neurodegener. 3, 19 (2008).
McCormack, A. L. et al. α-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS ONE 5, e12122 (2010).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Ebrahimi-Fakhari, D., Wahlster, L. & McLean, P. J. Molecular chaperones in Parkinson's disease—present and future. J. Parkinsons Dis. 1, 299–320 (2011).
Plowey, E. D. & Chu, C. T. Synaptic dysfunction in genetic models of Parkinson's disease: a role for autophagy? Neurobiol. Dis. 43, 60–67 (2011).
Cuervo, A. M. Autophagy: many paths to the same end. Mol. Cell Biochem. 263, 55–72 (2004).
Orenstein, S. J. et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 16, 394–406 (2013).
Cook, C., Stetler, C. & Petrucelli, L. Disruption of protein quality control in Parkinson's disease. Cold Spring Harbor Perspect. Med. 2, a009423 (2012).
Matsuda, W. et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 29, 444–453 (2009).
Luk, K. C. et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Kasai, H., Takahashi, N. & Tokumaru, H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol. Rev. 92, 1915–1964 (2012).
Jahn, R. & Fasshauer, D. Molecular machines governing exocytosis of synaptic vesicles. Nature 490, 201–207 (2012).
Burgoyne, R. D. & Morgan, A. Chaperoning the SNAREs: a role in preventing neurodegeneration? Nat. Cell Biol. 13, 8–9 (2011).
Westphal, C. H. & Chandra, S. S. Monomeric synucleins generate membrane curvature. J. Biol. Chem. 288, 1829–1840 (2013).
McPherson, P. S. et al. A presynaptic inositol-5-phosphatase. Nature 379, 353–357 (1996).
Milosevic, I. et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 72, 587–601 (2011).
Bonifacino, J. S. & Hurley, J. H. Retromer. Curr. Opin. Cell Biol. 20, 427–436 (2008).
Shi, A. et al. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J. 28, 3290–3302 (2009).
Seaman, M. N. Recycle your receptors with retromer. Trends Cell Biol. 15, 68–75 (2005).
Schneider, A. & Simons, M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 352, 33–47 (2012).
Braschi, E. et al. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr. Biol. 20, 1310–1315 (2010).
Seaman, M. N. The retromer complex—endosomal protein recycling and beyond. J. Cell Sci. 125, 4693–4702 (2012).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Sheerin, U. M. et al. Screening for VPS35 mutations in Parkinson's disease. Neurobiol. Aging 33, 838.e1–838.e5 (2012).
Edwards, A. W. G. H. Hardy (1908) and Hardy–Weinberg equilibrium. Genetics 179, 1143–1150 (2008).
Saad, M. et al. Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson's disease in the European population. Hum. Mol. Genet. 20, 615–627 (2011).
Edwards, T. L. et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet. 74, 97–109 (2010).
Wijsman, E. M. The role of large pedigrees in an era of high-throughput sequencing. Hum. Genet. 131, 1555–1563 (2012).
Krebs, C. E. & Paisán-Ruíz, C. The use of next-generation sequencing in movement disorders. Front. Genet. 3, 75 (2012).
Toft, M. et al. Parkinsonism, FXTAS, and FMR1 premutations. Mov. Disord. 20, 230–233 (2005).
Baba, Y., Uitti, R. J., Farrer, M. J. & Wszolek, Z. K. Atypical parkinsonism and SCA8. Parkinsonism Relat. Disord. 12, 396 (2006).
Furtado, S. et al. Profile of families with parkinsonism-predominant spinocerebellar ataxia type 2 (SCA2). Mov. Disord. 19, 622–629 (2004).
Gwinn-Hardy, K. et al. Spinocerebellar ataxia type 3 phenotypically resembling Parkinson disease in a black family. Arch. Neurol. 58, 296–299 (2001).
Nalls, M. A. et al. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet 377, 641–649 (2011).
Lill, C. M. et al. Comprehensive research synopsis and systematic meta-analyses in Parkinson's disease genetics: the PDGene database. PLoS Genet. 8, e1002548 (2012).
Acknowledgements
The work of the authors is funded by the Leading Edge Endowment Fund (to J. Trinh), and through a Canada Excellence Research Chair and the Don Rix Chair in Genetic Medicine (to M. Farrer).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article and made substantial contributions to discussion of the content, writing of the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Trinh, J., Farrer, M. Advances in the genetics of Parkinson disease. Nat Rev Neurol 9, 445–454 (2013). https://doi.org/10.1038/nrneurol.2013.132
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.132
This article is cited by
-
The small GTPase Rit2 modulates LRRK2 kinase activity, is required for lysosomal function and protects against alpha-synuclein neuropathology
npj Parkinson's Disease (2023)
-
Dopaminergic Dysfunction and Glucose Metabolism Characteristics in Parkin-Induced Early-Onset Parkinson’s Disease Compared to Genetically Undetermined Early-Onset Parkinson’s Disease
Phenomics (2023)
-
Evaluation of the PREDIGT score’s performance in identifying newly diagnosed Parkinson’s patients without motor examination
npj Parkinson's Disease (2022)
-
PINK1 kinase dysfunction triggers neurodegeneration in the primate brain without impacting mitochondrial homeostasis
Protein & Cell (2022)
-
Downregulating expression of OPTN elevates neuroinflammation via AIM2 inflammasome- and RIPK1-activating mechanisms in APP/PS1 transgenic mice
Journal of Neuroinflammation (2021)