Abstract
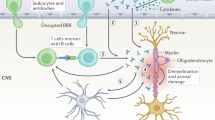
Myelin oligodendrocyte glycoprotein (MOG) has been identified as a target of demyelinating autoantibodies in animal models of inflammatory demyelinating diseases of the CNS, such as multiple sclerosis (MS). Numerous studies have aimed to establish a role for MOG antibodies in patients with MS, although the results have been controversial. Cell-based immunoassays using MOG expressed in mammalian cells have demonstrated the presence of high-titre MOG antibodies in paediatric patients with acute disseminated encephalomyelitis, MS, aquaporin-4-seronegative neuromyelitis optica, or isolated optic neuritis or transverse myelitis, but only rarely in adults with these disorders. These studies indicate that MOG antibodies could be associated with a broad spectrum of acquired human CNS demyelinating diseases. This Review article discusses the current literature on MOG antibodies, their potential clinical relevance, and their role in the pathogenesis of MOG antibody-associated demyelinating disorders.
Key Points
-
Myelin oligodendrocyte glycoprotein (MOG) is a target of demyelinating autoantibodies in animal models of inflammatory demyelinating diseases of the CNS
-
Studies using cell-based immunoassays have identified MOG antibodies in human inflammatory demyelinating CNS diseases
-
High-titre MOG antibodies are present in paediatric patients with acute disseminated encephalomyelitis, multiple sclerosis (MS), aquaporin-4-seronegative neuromyelitis optica, or isolated optic neuritis or transverse myelitis
-
High-titre MOG antibodies are only rarely seen in adults with these demyelinating CNS disorders
-
MOG antibodies might be associated with a broad spectrum of acquired human CNS demyelinating diseases
-
The clinical presentation observed in anti-MOG-positive patients resembles the disease features in experimental animal models more closely than the clinical presentation of patients with MS
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Popescu, B. F. & Lucchinetti, C. F. Pathology of demyelinating diseases. Annu. Rev. Pathol. 7, 185–217 (2012).
Conrad, K., Roggenbuck, D., Reinhold, D. & Sack, U. Autoantibody diagnostics in clinical practice. Autoimmun. Rev. 11, 207–211 (2012).
Reindl, M., Khalil, M. & Berger, T. Antibodies as biological markers for pathophysiological processes in MS. J. Neuroimmunol. 180, 50–62 (2006).
Cross, A. H. & Waubant, E. MS and the B cell controversy. Biochim. Biophys. Acta 1812, 231–238 (2011).
Krumbholz, M., Derfuss, T., Hohlfeld, R. & Meinl, E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat. Rev. Neurol. 8, 613–623 (2012).
Mayer, M. C. & Meinl, E. Glycoproteins as targets of autoantibodies in CNS inflammation: MOG and more. Ther. Adv. Neurol. Disord. 5, 147–159 (2012).
Pham-Dinh, D. et al. Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex. Proc. Natl Acad. Sci. USA 90, 7990–7994 (1993).
Pham-Dinh, D. et al. Characterization and expression of the cDNA coding for the human myelin/oligodendrocyte glycoprotein. J. Neurochem. 63, 2353–2356 (1994).
Brunner, C., Lassmann, H., Waehneldt, T. V., Matthieu, J. M. & Linington, C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase in the CNS of adult rats. J. Neurochem. 52, 296–304 (1989).
Johns, T. G. & Bernard, C. C. The structure and function of myelin oligodendrocyte glycoprotein. J. Neurochem. 72, 1–9 (1999).
Lebar, R., Lubetzki, C., Vincent, C., Lombrail, P. & Boutry, J. M. The M2 autoantigen of central nervous system myelin, a glycoprotein present in oligodendrocyte membrane. Clin. Exp. Immunol. 66, 423–434 (1986).
Schluesener, H. J., Sobel, R. A., Linington, C. & Weiner, H. L. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J. Immunol. 139, 4016–4021 (1987).
Linington, C., Bradl, M., Lassmann, H., Brunner, C. & Vass, K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 130, 443–454 (1988).
Iglesias, A., Bauer, J., Litzenburger, T., Schubart, A. & Linington, C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 36, 220–234 (2001).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Krishnamoorthy, G., Lassmann, H., Wekerle, H. & Holz, A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J. Clin. Invest. 116, 2385–2392 (2006).
Bettelli, E., Baeten. D., Jäger. A., Sobel, R. A., Kuchroo. V. K. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J. Clin. Invest. 116, 2393–2402 (2006).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
von Budingen, H. C. et al. Frontline: epitope recognition on the myelin/oligodendrocyte glycoprotein differentially influences disease phenotype and antibody effector functions in autoimmune demyelination. Eur. J. Immunol. 34, 2072–2083 (2004).
Marta, C. B., Oliver, A. R., Sweet, R. A., Pfeiffer, S. E. & Ruddle, N. H. Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology. Proc. Natl Acad. Sci. USA 102, 13992–13997 (2005).
Lalive, P. H. et al. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc. Natl Acad. Sci. USA 103, 2280–2285 (2006).
Zhou, D. et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc. Natl Acad. Sci. USA 103, 19057–19062 (2006).
O'Connor, K. C. et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 13, 211–217 (2007).
McLaughlin, K. A. et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J. Immunol. 183, 4067–4076 (2009).
Brilot, F. et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann. Neurol. 66, 833–842 (2009).
Selter, R. C. et al. Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology 74, 1711–1715 (2010).
Di Pauli, F. et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin. Immunol. 138, 247–254 (2011).
Lalive, P. H. et al. Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult. Scler. 17, 297–302 (2011).
Probstel, A. K. et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology 77, 580–588 (2011).
Mader, S. et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J. Neuroinflamm. 8, 184 (2011).
Rostásy, K. et al. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch. Neurol. 69, 752–756 (2012).
Kitley, J. et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 79, 1273–1277 (2012).
Rostásy, K. et al. Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult. Scler. http://dx.doi.org/10.1177/1352458512470310.
Storch, M. K. et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 8, 681–694 (1998).
Sakuma, H. et al. Clinicopathological study of a myelin oligodendrocyte glycoprotein-induced demyelinating disease in LEW.1AV1 rats. Brain 127, 2201–2213 (2004).
Young, N. P. et al. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain 133, 333–348 (2010).
Burrer, R. et al. Exacerbated pathology of viral encephalitis in mice with central nervous system-specific autoantibodies. Am. J. Pathol. 170, 557–566 (2007).
Bennett, J. L. et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 66, 617–629 (2009).
Bradl, M. et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann. Neurol. 66, 630–643 (2009).
Saadoun, S. et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349–361 (2010).
Young, N. P., Weinshenker, B. G. & Lucchinetti, C. F. Acute disseminated encephalomyelitis: current understanding and controversies. Semin. Neurol. 28, 84–94 (2008).
Krupp, L. B., Banwell, B., Tenembaum, S. & International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 68 (Suppl. 2), S7–S12 (2007).
Huppke, P. et al. Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult. Scler. 19, 941–946 (2013).
Renoux, C. et al. Natural history of multiple sclerosis with childhood onset. N. Engl. J. Med. 356, 2603–2613 (2007).
Lennon, V. A. et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004).
Lennon, V. A., Kryzer, T. J., Pittock, S. J., Verkman, A. S. & Hinson, S. R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005).
Wingerchuk, D. M., Lennon, V. A., Pittock, S. J., Lucchinetti, C. F. & Weinshenker, B. G. Revised diagnostic criteria for neuromyelitis optica. Neurology 66, 1485–1489 (2006).
Waters, P. J. et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 78, 665–671 (2012).
Marignier, R. et al. Aquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entity. Neurology http://dx.doi.org/10.1212/WNL.0b013e318296e917.
Acknowledgements
The authors' research is funded by grants W1206 and I916 from the Austrian Science Fund (FWF; M. Reindl) and grant 14158 from the Jubilaeumsfonds of the Austrian National Bank (K. Rostásy).
Author information
Authors and Affiliations
Contributions
M. Reindl, F. Di Pauli, K. Rostásy and T. Berger contributed equally to researching data for the article, discussion of the content, writing, and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
A comparison of methods using human MOG expressed in mammalian cells for the detection of serum MOG-IgG (DOC 61 kb)
Rights and permissions
About this article
Cite this article
Reindl, M., Di Pauli, F., Rostásy, K. et al. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol 9, 455–461 (2013). https://doi.org/10.1038/nrneurol.2013.118
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.118
This article is cited by
-
Advancements in Exploring Metal-Organic Gels: Structure, Synthesis, and Characterization with a Focus on Preparation Conditions
Journal of Cluster Science (2024)
-
Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS): contemporary advances and current controversies
Journal of Neurology (2024)
-
Proteomic profiling of cerebrospinal fluid in pediatric myelin oligodendrocyte glycoprotein antibody-associated disease
World Journal of Pediatrics (2024)
-
MRI features of myelin oligodendrocyte glycoprotein antibody disease: a descriptive study—how it differs from neuromyelitis optica spectrum disorders and multiple sclerosis
Egyptian Journal of Radiology and Nuclear Medicine (2023)
-
Emerging Principles for Treating Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD)
Current Treatment Options in Neurology (2023)