Abstract
People over the age of 90 years—the oldest old—are the fastest growing sector of the population. A substantial proportion of these individuals are affected by dementia, with major implications for the individual as well as society. Research on dementia in the oldest old is important for service planning, and the absence of dementia at this exceptional old age may serve as a model of successful ageing. This Review summarizes population-based epidemiological studies of dementia and its underlying neuropathology in nonagenarians and centenarians. The available data, although somewhat limited, show an age-specific and sex-specific profile of dementia status in very late life, resulting from a variety of neuropathologies that often co-occur. Extensive overlap in neuropathology between cognitively normal and cognitively impaired individuals is evident despite challenges to gathering data particular to this population. A complex picture is emerging of multiple pathogenetic mechanisms underlying dementia, and of the potential risk and protective factors for dementia that interact with genetics and lifestyle in normal and exceptional cognitive ageing.
Key Points
-
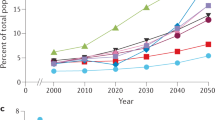
People over the age of 90 years (the oldest old) are the fastest growing sector of the population, with a substantial proportion developing dementia
-
The prevalence of dementia is age-specific: rates increase from about 25–30% in those in their early 90s to about 50% in the late 90s and 60% in centenarians
-
Prevalence of dementia is lower in men than in women, but the incidence at 90 years or over does not differ by sex, suggesting shorter survival time in men
-
Multiple neuropathologies underlie dementia, including Alzheimer disease neuropathological change and vascular pathology, which often co-occur, as well as Lewy-related pathology, hippocampal sclerosis and cerebral amyloid angiopathy
-
Diffuse neocortical neurofibrillary tangles and neocortical and hippocampal atrophy are the most consistent correlates of dementia in the oldest old
-
Neuropathology is common in cognitively normal individuals aged over 90 years, and better markers are needed to distinguish dementia from normal cognitive ageing
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Miller, L. S. et al. Cognitive performance in centenarians and the oldest old: norms from the Georgia Centenarian Study. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 17, 575–590 (2010).
Corrada, M. M., Berlau, D. J. & Kawas, C. H. A population-based clinicopathological study in the oldest old: the 90+ study. Curr. Alzheimer Res. 9, 709–717 (2012).
Census of Population and Housing. Australian Bureau of Statistics [online], (2011).
Population Projections, Australia, 2006 to 2101. Australian Bureau of Statistics [online], (2011).
Richmond, R. L. The changing face of the Australian population: growth in centenarians. Med. J. Aust. 188, 720–723 (2008).
Sachdev, P. S., Levitan, C. & Crawford, J. D. Methodological issues in centenarian research: pitfalls and challenges. Asian J. Gerontol. Geriatr. 7, 44–48 (2012).
Calvert, J. F. Jr, Hollander-Rodriguez, J., Kaye, J. & Leahy, M. Dementia-free survival among centenarians: an evidence-based review. J. Gerontol. A Biol. Sci. Med. Sci. 61, 951–956 (2006).
Leahy, M. J., Thurber, D. & Calvert, J. F. Jr. Benefits and challenges of research with the oldest old for participants and nurses. Geriatr. Nurs. 26, 21–28 (2005).
Poon, L. W. et al. Understanding dementia prevalence among centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 67, 358–365 (2012).
Andersen-Ranberg, K., Schroll, M. & Jeune, B. Healthy centenarians do not exist, but autonomous centenarians do: a population-based study of morbidity among Danish centenarians. J. Am. Geriatr. Soc. 49, 900–908 (2001).
Erkinjuntti, T., Ostbye, T., Steenhuis, R. & Hachinski, V. The effect of different diagnostic criteria on the prevalence of dementia. N. Engl. J. Med. 337, 1667–1674 (1997).
Pioggiosi, P. et al. Different classification systems yield different dementia occurrence among nonagenarians and centenarians. Dement. Geriatr. Cogn. Disord. 17, 35–41 (2004).
Zaccai, J., Ince, P. & Brayne, C. Population-based neuropathological studies of dementia: design, methods and areas of investigation–a systematic review. BMC Neurol. 6, 2 (2006).
Nelson, P. T. et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381 (2012).
White, L. et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu–Asia Aging Study. J. Geriatr. Psychiatry Neurol. 18, 224–227 (2005).
van Vliet, P., Oleksik, A. M., Mooijaart, S. P., de Craen, A. J. & Westendorp, R. G. APOE genotype modulates the effect of serum calcium levels on cognitive function in old age. Neurology 72, 821–828 (2009).
Andersen, K. et al. Cognitive impairment and mortality among nonagenarians: the Danish 1905 cohort survey. Dement. Geriatr. Cogn. Disord. 13, 156–163 (2002).
Börjesson-Hanson, A., Edin, E., Gislason, T. & Skoog, I. The prevalence of dementia in 95 year olds. Neurology 63, 2436–2438 (2004).
Corrada, M. M., Brookmeyer, R., Berlau, D., Paganini-Hill, A. & Kawas, C. H. Prevalence of dementia after age 90: results from the 90+ study. Neurology 71, 337–343 (2008).
Riedel-Heller, S. G., Busse, A., Aurich, C., Matschinger, H. & Angermeyer, M. C. Prevalence of dementia according to DSM-III-R and ICD-10: results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Part 1. Br. J. Psychiatry 179, 250–254 (2001).
von Strauss, E., Viitanen, M., De Ronchi, D., Winblad, B. & Fratiglioni, L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch. Neurol. 56, 587–592 (1999).
Ebly, E. M., Parhad, I. M., Hogan, D. B. & Fung, T. S. Prevalence and types of dementia in the very old: results from the Canadian Study of Health and Aging. Neurology 44, 1593–1600 (1994).
Wernicke, T. F. & Reischies, F. M. Prevalence of dementia in old age: clinical diagnoses in subjects aged 95 years and older. Neurology 44, 250–253 (1994).
Savva, G. M. et al. Age, neuropathology, and dementia. N. Engl. J. Med. 360, 2302–2309 (2009).
Polvikoski, T. et al. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology 56, 1690–1696 (2001).
Ott, A. et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ 310, 970–973 (1995).
Chiu, H. F. et al. Prevalence of dementia in Chinese elderly in Hong Kong. Neurology 50, 1002–1009 (1998).
Zhao, J. et al. The oldest old in the last year of life: population-based findings from Cambridge City over-75s Cohort Study participants aged 85 and older at death. J. Am. Geriatr. Soc. 58, 1–11 (2010).
Samuelsson, S. M. et al. The Swedish Centenarian Study: a multidisciplinary study of five consecutive cohorts at the age of 100. Int. J. Aging Hum. Dev. 45, 223–253 (1997).
Ravaglia, G. et al. Prevalence and severity of dementia among northern Italian centenarians. Neurology 53, 416–418 (1999).
Gondo, Y. et al. Functional status of centenarians in Tokyo, Japan: developing better phenotypes of exceptional longevity. J. Gerontol. A Biol. Sci. Med. Sci. 61, 305–310 (2006).
Kliegel, M., Moor, C. & Rott, C. Cognitive status and development in the oldest old: a longitudinal analysis from the Heidelberg Centenarian Study. Arch. Gerontol. Geriatr. 39, 143–156 (2004).
Andersen-Ranberg, K., Vasegaard, L. & Jeune, B. Dementia is not inevitable: a population-based study of Danish centenarians. J. Gerontol. B Psychol. Sci. Soc. Sci. 56, P152–P159 (2001).
Silver, M. H., Newell, K., Brady, C., Hedley-White, E. T. & Perls, T. T. Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom. Med. 64, 493–501 (2002).
Choi, Y.-H. et al. Distributions of ACE and APOE polymorphisms and their relations with dementia status in Korean centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 58, M227–M231 (2003).
Sobel, E. et al. Lack of association of apolipoprotein E allele ε4 with late-onset Alzheimer's disease among Finnish centenarians. Neurology 45, 903–907 (1995).
Sachdev, P. S. et al. The Sydney Centenarian Study: methodology and profile of centenarians and near-centenarians. Int. Psychogeriatr. 25, 993–1005 (2013).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd edn, revised 103–107 (American Psychiatric Association, Washington, DC, 1987).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn 133–155 (American Psychiatric Association, Washington, DC, 1994).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Ritchie, K. Mental status examination of an exceptional case of longevity J. C. aged 118 years. Br. J. Psychiatry 166, 229–235 (1995).
den Dunnen, W. F. A. et al. No disease in the brain of a 115-year-old woman. Neurobiol. Aging 29, 1127–1132 (2008).
Corrada, M. M., Brookmeyer, R., Paganini-Hill, A., Berlau, D. & Kawas, C. H. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann. Neurol. 67, 114–121 (2010).
Miech, R. A. et al. Incidence of AD may decline in the early 90s for men, later for women: the Cache County study. Neurology 58, 209–218 (2002).
Ruitenberg, A., Ott, A., van Swieten, J. C., Hofman, A. & Breteler, M. M. Incidence of dementia: does gender make a difference? Neurobiol. Aging 22, 575–580 (2001).
Copeland, J. R. et al. Undifferentiated dementia, Alzheimer's disease and vascular dementia: age- and gender-related incidence in Liverpool. The MRC–ALPHA Study. Br. J. Psychiatry 175, 433–438 (1999).
Hall, C. B. et al. Dementia incidence may increase more slowly after age 90: results from the Bronx Aging Study. Neurology 65, 882–886 (2005).
Edland, S. D., Rocca, W. A., Petersen, R. C., Cha, R. H. & Kokmen, E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch. Neurol. 59, 1589–1593 (2002).
The Canadian Study of Health and Aging Working Group. The incidence of dementia in Canada. Neurology 55, 66–73 (2000).
Fichter, M. M., Schroppel, H. & Meller, I. Incidence of dementia in a Munich community sample of the oldest old. Eur. Arch. Psychiatry Clin. Neurosci. 246, 320–328 (1996).
Fries, J. F. Aging, natural death, and the compression of morbidity. N. Engl. J. Med. 303, 130–135 (1980).
Andersen, S. L., Sebastiani, P., Dworkis, D. A., Feldman, L. & Perls, T. T. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J. Gerontol. A Biol. Sci. Med. Sci. 67, 395–405 (2012).
Schaie, K. W., Willis, S. L. & Caskie, G. I. The Seattle longitudinal study: relationship between personality and cognition. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 11, 304–324 (2004).
Singer, T., Verhaeghen, P., Ghisletta, P., Lindenberger, U. & Baltes, P. B. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE). Psychol. Aging 18, 318–331 (2003).
Witthaus, E., Ott, A., Barendregt, J. J., Breteler, M. & Bonneux, L. Burden of mortality and morbidity from dementia. Alzheimer Dis. Assoc. Disord. 13, 176–181 (1999).
Hassing, L. B. et al. Terminal decline and markers of cerebro- and cardiovascular disease: findings from a longitudinal study of the oldest old. J. Gerontol. B Psychol. Sci. Soc. Sci. 57, P268–P276 (2002).
Helmer, C., Joly, P., Letenneur, L., Commenges, D. & Dartigues, J. F. Mortality with dementia: results from a French prospective community-based cohort. Am. J. Epidemiol. 154, 642–648 (2001).
Neale, R., Brayne, C. & Johnson, A. L. Cognition and survival: an exploration in a large multicentre study of the population aged 65 years and over. Int. J. Epidemiol. 30, 1383–1388 (2001).
Moschetti, K., Cummings, P. L., Sorvillo, F. & Kuo, T. Burden of Alzheimer's disease-related mortality in the United States, 1999–2008. J. Am. Geriatr. Soc. 60, 1509–1514 (2012).
Wolfson, C. et al. A reevaluation of the duration of survival after the onset of dementia. N. Engl. J. Med. 344, 1111–1116 (2001).
Wakisaka, Y. et al. Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol. 106, 374–382 (2003).
Masuda, J., Tanaka, K., Ueda, K. & Omae, T. Autopsy study of incidence and distribution of cerebral amyloid angiopathy in Hisayama, Japan. Stroke 19, 205–210 (1988).
Oinas, M. et al. Neuropathologic findings of dementia with Lewy bodies (DLB) in a population-based Vantaa 85+ study. J. Alzheimers Dis. 18, 677–689 (2009).
Barkhof, F. et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology 69, 1521–1527 (2007).
Tanskanen, M. et al. Prevalence and severity of cerebral amyloid angiopathy: a population-based study on very elderly Finns (Vantaa 85+). Neuropathol. Appl. Neurobiol. 38, 329–336 (2012).
Tanskanen, M. et al. Cerebral amyloid angiopathy in a 95+ cohort: complement activation and apolipoprotein E (ApoE) genotype. Neuropathol. Appl. Neurobiol. 31, 589–599 (2005).
Zaccai, J. et al. Patterns and stages of α-synucleinopathy: relevance in a population-based cohort. Neurology 70, 1042–1048 (2008).
Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 357, 169–175 (2001).
White, L. Brain lesions at autopsy in older Japanese–American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu–Asia Aging Study. J. Alzheimers Dis. 18, 713–725 (2009).
Pfeifer, L. A., White, L. R., Ross, G. W., Petrovitch, H. & Launer, L. J. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology 58, 1629–1634 (2002).
Robinson, J. L. et al. Neocortical and hippocampal amyloid-β and tau measures associate with dementia in the oldest-old. Brain 134, 3708–3715 (2011).
Nelson, P. T. et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 134, 1506–1518 (2011).
Matthews, F. E. et al. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 6, e1000180 (2009).
Nelson, P. T. et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J. Neuropathol. Exp. Neurol. 68, 774–784 (2009).
Jellinger, K. A. & Attems, J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 113, 107–117 (2007).
Janocko, N. J. et al. Neuropathologically defined subtypes of Alzheimer's disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol. 124, 681–692 (2012).
Ding, Z. T. et al. Argyrophilic grain disease: frequency and neuropathology in centenarians. Acta Neuropathol. 111, 320–328 (2006).
Ferrer, I., Santpere, G. & van Leeuwen, F. W. Argyrophilic grain disease. Brain 131, 1416–1432 (2008).
[No authors listed] Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol. Aging 18, S1–S2 (1997).
Fujimi, K. et al. Clinicopathological outline of dementia with Lewy bodies applying the revised criteria: the Hisayama study. Brain Pathol. 18, 317–325 (2008).
Nelson, P. T. et al. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 121, 571–587 (2011).
Jellinger, K. A. & Attems, J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 119, 421–433 (2010).
Keage, H. A. et al. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol. 9, 3 (2009).
Wharton, S. B. et al. Epidemiological neuropathology: the MRC Cognitive Function and Aging Study experience. J. Alzheimers Dis. 25, 359–372 (2011).
James, B. D., Bennett, D. A., Boyle, P. A., Leurgans, S. & Schneider, J. A. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA 307, 1798–1800 (2012).
Jellinger, K. A. & Attems, J. Prevalence and pathology of vascular dementia in the oldest-old. J. Alzheimers Dis. 21, 1283–1293 (2010).
Ellis, R. J. et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, Part XV. Neurology 46, 1592–1596 (1996).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Middleton, L. E., Grinberg, L. T., Miller, B., Kawas, C. & Yaffe, K. Neuropathologic features associated with Alzheimer disease diagnosis: age matters. Neurology 77, 1737–1744 (2011).
Mirra, S. S. et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41, 479–486 (1991).
Haroutunian, V. et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch. Neurol. 65, 1211–1217 (2008).
Prohovnik, I. et al. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology 66, 49–55 (2006).
Murray, M. E. et al. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 10, 785–796 (2011).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Nelson, P. T. et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J. Neuropathol. Exp. Neurol. 66, 1136–1146 (2007).
Riley, K. P. et al. Prediction of preclinical Alzheimer's disease: longitudinal rates of change in cognition. J. Alzheimers Dis. 25, 707–717 (2011).
Driscoll, I. et al. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann. Neurol. 60, 688–695 (2006).
Bennett, D. A. et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844 (2006).
Balasubramanian, A. B., Kawas, C. H., Peltz, C. B., Brookmeyer, R. & Corrada, M. M. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology 79, 915–921 (2012).
Pantoni, L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701 (2010).
Vermeer, S. E., Longstreth, W. T. Jr & Koudstaal, P. J. Silent brain infarcts: a systematic review. Lancet Neurol. 6, 611–619 (2007).
Smith, E. E., Schneider, J. A., Wardlaw, J. M. & Greenberg, S. M. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 11, 272–282 (2012).
Debette, S. & Markus, H. S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341, c3666 (2010).
Dolan, D. et al. Age, Alzheimer's disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain 133, 2225–2231 (2010).
Sinka, L. et al. Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest-old. J. Neuropathol. Exp. Neurol. 69, 1247–1255 (2010).
Piguet, O. et al. Are MRI white matter lesions clinically significant in the 'old-old'? Evidence from the Sydney Older Persons Study. Dement. Geriatr. Cogn. Disord. 15, 143–150 (2003).
Okamoto, Y. et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 123, 381–394 (2012).
Hyman, B. T. et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 8, 1–13 (2012).
Montine, T. J. et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 123, 1–11 (2012).
Terry, R. D. & Katzman, R. Life span and synapses: will there be a primary senile dementia? Neurobiol. Aging 22, 347–348 (2001).
Freeman, S. H. et al. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J. Neuropathol. Exp. Neurol. 67, 1205–1212 (2008).
Iacono, D. et al. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology 73, 665–673 (2009).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Head, E. et al. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol. Aging 30, 1125–1134 (2009).
Honer, W. G. et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl. Psychiatry 2, e114 (2012).
Kawas, C. H. The oldest old and the 90+ Study. Alzheimers Dement. 4, S56–S59 (2008).
Polvikoski, T. M. et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology 75, 2071–2078 (2010).
Beeri, M. S. et al. Memory activation in healthy nonagenarians. Neurobiol. Aging 32, 515–523 (2011).
Kawas, C. H. et al. Amyloid imaging and cognitive decline in nondemented oldest-old: the 90+ Study. Alzheimer's Dement. 9, 199–203 (2013).
Sebastiani, P. et al. Genetic signatures of exceptional longevity in humans. PLoS ONE 7, e29848 (2012).
Perls, T. T. et al. Life-long sustained mortality advantage of siblings of centenarians. Proc. Natl Acad. Sci. USA 99, 8442–8447 (2002).
Lee, T., Henry, J. D., Trollor, J. N. & Sachdev, P. S. Genetic influences on cognitive functions in the elderly: a selective review of twin studies. Brain Res. Rev. 64, 1–13 (2010).
Christensen, K., Johnson, T. E. & Vaupel, J. W. The quest for genetic determinants of human longevity: challenges and insights. Nat. Rev. Genet. 7, 436–448 (2006).
Kamboh, M. I. et al. Genome-wide association study of Alzheimer's disease. Transl. Psychiatry 2, e117 (2012).
Reitz, C., Brayne, C. & Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 7, 137–152 (2011).
Kamboh, M. I. et al. Genome-wide association analysis of age-at-onset in Alzheimer's disease. Mol. Psychiatry 17, 1340–1346 (2012).
Corrada, M. M., Paganini-Hill, A., Berlau, D. J. & Kawas, C. H. Apolipoprotein E genotype, dementia, and mortality in the oldest old: the 90+ Study. Alzheimers Dement. 9, 12–18 (2013).
Juva, K. et al. APOE ε4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology 54, 412–415 (2000).
Skoog, I. et al. A population study of apoE genotype at the age of 85: relation to dementia, cerebrovascular disease, and mortality. J. Neurol. Neurosurg. Psychiatry 64, 37–43 (1998).
Qiu, C., Kivipelto, M., Aguero-Torres, H., Winblad, B. & Fratiglioni, L. Risk and protective effects of the APOE gene towards Alzheimer's disease in the Kungsholmen project: variation by age and sex. J. Neurol. Neurosurg. Psychiatry 75, 828–833 (2004).
Berlau, D. J., Corrada, M. M., Head, E. & Kawas, C. H. APOE ε2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology 72, 829–834 (2009).
Peuralinna, T. et al. APOE and AβPP gene variation in cortical and cerebrovascular amyloid-β pathology and Alzheimer's disease: a population-based analysis. J. Alzheimers Dis. 26, 377–385 (2011).
Mahley, R. W., Weisgraber, K. H. & Huang, Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl Acad. Sci. USA 103, 5644–5651 (2006).
Peltz, C. B., Corrada, M. M., Berlau, D. J. & Kawas, C. H. Cognitive impairment in nondemented oldest-old: prevalence and relationship to cardiovascular risk factors. Alzheimers Dement. 8, 87–94 (2012).
Sachdev, P. S. et al. Risk profiles for mild cognitive impairment vary by age and sex: the Sydney Memory and Ageing Study. Am. J. Geriatr. Psychiatry 20, 854–865 (2012).
Skoog, I. et al. 15-year longitudinal study of blood pressure and dementia. Lancet 347, 1141–1145 (1996).
Li, G. et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J. Am. Geriatr. Soc. 55, 1161–1167 (2007).
Molander, L., Gustafson, Y. & Lovheim, H. Longitudinal associations between blood pressure and dementia in the very old. Dement. Geriatr. Cogn. Disord. 30, 269–276 (2010).
Ruitenberg, A. et al. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement. Geriatr. Cogn. Disord. 12, 33–39 (2001).
Verghese, J., Lipton, R. B., Hall, C. B., Kuslansky, G. & Katz, M. J. Low blood pressure and the risk of dementia in very old individuals. Neurology 61, 1667–1672 (2003).
Mielke, M. M. et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64, 1689–1695 (2005).
Reitz, C., Tang, M. X., Luchsinger, J. & Mayeux, R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 61, 705–714 (2004).
Bullain, S. S. et al. Poor physical performance and dementia in the oldest old: the 90+ Study. JAMA Neurol. 70, 107–113 (2013).
Peltz, C. B., Corrada, M. M., Berlau, D. J. & Kawas, C. H. Incidence of dementia in oldest-old with amnestic MCI and other cognitive impairments. Neurology 77, 1906–1912 (2011).
Wilson, R. S., Segawa, E., Boyle, P. A. & Bennett, D. A. Influence of late-life cognitive activity on cognitive health. Neurology 78, 1123–1129 (2012).
Scarmeas, N., Levy, G., Tang, M. X., Manly, J. & Stern, Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 57, 2236–2242 (2001).
Stern, Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 11, 1006–1012 (2012).
World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders. Clinical Descriptions and Diagnostic Guidelines (WHO, Geneva, 1992).
Launer, L. J., Hughes, T. M. & White, L. R. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann. Neurol. 70, 774–780 (2011).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65, 1863–1872 (2005).
Halliday, G. M., Holton, J. L., Revesz, T. & Dickson, D. W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 122, 187–204 (2011).
Hamilton, R. L. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol. 10, 378–384 (2000).
Brundel, M., de Bresser, J., van Dillen, J. J., Kappelle, L. J. & Biessels, G. J. Cerebral microinfarcts: a systematic review of neuropathological studies. J. Cereb. Blood Flow Metab. 32, 425–436 (2012).
Acknowledgements
This work was supported by the National Health & Medical Research Council (NHMRC) of Australia (Project Grant 630593 and Program Grant 568969). M. J. Slavin was supported by Dementia Collaborative Research Centre—Assessment and Better Care funding and the NHMRC as part of an Australian Government Initiative. Z. Yang is supported by funding from the China Scholarship Council for her Ph.D. candidature. We thank Mrs Angela Russell for editorial assistance.
Author information
Authors and Affiliations
Contributions
Z. Yang researched the data for the article and wrote the manuscript. All three authors provided substantial contributions to the discussion of the content and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Characteristics of population-based prevalence and incidence studies (DOC 28 kb)
Rights and permissions
About this article
Cite this article
Yang, Z., Slavin, M. & Sachdev, P. Dementia in the oldest old. Nat Rev Neurol 9, 382–393 (2013). https://doi.org/10.1038/nrneurol.2013.105
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.105
This article is cited by
-
Effects of kidney function, serum albumin and hemoglobin on dementia severity in the oldest old people with newly diagnosed Alzheimer’s disease in a residential aged care facility: a cross-sectional study
BMC Geriatrics (2020)
-
Third follow-up of the Cardiovascular Risk Factors, Aging and Dementia (CAIDE) cohort investigating determinants of cognitive, physical, and psychosocial wellbeing among the oldest old: the CAIDE85+ study protocol
BMC Geriatrics (2020)
-
Ocular biomarkers for cognitive impairment in nonagenarians; a prospective cross-sectional study
BMC Geriatrics (2020)
-
The puzzle of preserved cognition in the oldest old
Neurological Sciences (2020)
-
The 100-plus Study of cognitively healthy centenarians: rationale, design and cohort description
European Journal of Epidemiology (2018)