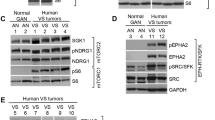
Abstract
Deficiency of the tumor suppressor protein merlin leads to the development of benign tumors of the nervous system such as schwannomas, ependymomas and meningiomas. These tumors can occur spontaneously or as part of a tumor predisposition syndrome called neurofibromatosis type 2 (NF2), which involves multiple tumors. Schwannomas are the hallmark tumors of NF2 and are the most frequent and well-characterized of the merlin-deficient tumors. Surgery or radiotherapy are used to treat single tumors and can leave the patient with substantial morbidity. Limitations of other treatment options for merlin-deficient tumors, such as the lack of effectiveness of chemotherapy, have led to an urgent requirement for new pharmaceutical therapies. Merlin-deficient tumors are genetically well-defined, which allows rational testing of new molecular therapies that have been developed and successfully used to treat various cancers in the past few years. This Review centers on four key families of receptor tyrosine kinases—the ErbB family, platelet-derived growth factor receptor β, insulin-like growth factor 1 receptor, and vascular endothelial growth factor receptors—focusing on their role in schwannoma pathobiology and the therapeutic potential of targeting these receptors and their downstream signaling pathways.
Key Points
-
Deficiency of the tumor suppressor protein merlin leads to a high burden of benign tumors—most commonly schwannomas, but also meningiomas and ependymomas
-
Emerging preclinical and clinical data suggest that prevention of tumor growth and survival by inhibiting the receptor tyrosine kinases ErbB2, platelet-derived growth factor receptor β, and insulin-like growth factor 1 receptor could be a promising therapeutic strategy
-
Drugs targeting vascular endothelial growth factor receptor signaling, which inhibit tumor vascularization, are also currently in phase II trials for schwannoma
-
Inhibiting multiple targets or a single downstream target that is common to several pathways may be the most effective approach and could help to limit adverse effects associated with long treatment times
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hanemann, C. O. & Evans, D. G. News on the genetics, epidemiology, medical care and translational research of schwannomas. J. Neurol. 253, 1533–1541 (2006).
Evans, D. G., Sainio, M. & Baser, M. E. Neurofibromatosis type 2. J. Med. Genet. 37, 897–904 (2000).
Tzahar, E. & Yarden, Y. The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands. Biochim. Biophys. Acta 1377, M25–M37 (1998).
Bange, J., Zwick, E. & Ullrich, A. Molecular targets for breast cancer therapy and prevention. Nat. Med. 7, 548–552 (2001).
Wickremesekera, A., Hovens, C. M. & Kaye, A. H. Expression of ErbB-1 and 2 in vestibular schwannomas. J. Clin. Neurosci. 14, 1199–1206 (2007).
Anglesio, M. S. et al. Mutation of ERBB2 provides a novel alternative mechanism for the ubiquitous activation of RAS-MAPK in ovarian serous low malignant potential tumors. Mol. Cancer Res. 6, 1678–1690 (2008).
Groenen, L. C., Nice, E. C. & Burgess, A. W. Structure-function relationships for the EGF/TGF-alpha family of mitogens. Growth Factors 11, 235–257 (1994).
Toyoda, H., Komurasaki, T., Ikeda, Y., Yoshimoto, M. & Morimoto, S. Molecular cloning of mouse epiregulin, a novel epidermal growth factor-related protein, expressed in the early stage of development. FEBS Lett. 377, 403–407 (1995).
Lonardo, F. et al. The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. New Biol. 2, 992–1003 (1990).
Beerli, R. R. et al. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol. Cell Biol. 15, 6496–6505 (1995).
Karunagaran, D. et al. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 15, 254–264 (15-1-1996).
Stern, D. F. ERBB3/HER3 and ERBB2/HER2 duet in mammary development and breast cancer. J. Mammary Gland Biol. Neoplasia 13, 215–223 (2008).
Garratt, A. N., Britsch, S. & Birchmeier, C. Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays 22, 987–996 (2000).
Birchmeier, C. & Nave, K. A. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497 (2008).
Shah, N. M., Marchionni, M. A., Isaacs, I., Stroobant, P. & Anderson, D. J. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell 77, 349–360 (1994).
Meyer, D. & Birchmeier, C. Multiple essential functions of neuregulin in development. Nature 378, 386–390 (1995).
Dong, Z. et al. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron 15, 585–596 (1995).
Syroid, D. E. et al. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc. Natl Acad. Sci. USA 93, 9229–9234 (1996).
Marchionni, M. A. et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature 362, 312–318 (1993).
Lee, K. F. et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378, 394–398 (1995).
Meier, C., Parmantier, E., Brennan, A., Mirsky, R. & Jessen, K. R. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J. Neurosci. 19, 3847–3859 (1999).
Riese, D. J., van Raaij, T. M., Plowman, G. D., Andrews, G. C. & Stern, D. F. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol. Cell Biol. 15, 5770–5776 (1995).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Beeser, A., Jaffer, Z. M., Hofmann, C. & Chernoff, J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J. Biol. Chem. 280, 36609–36615 (2005).
Prenzel, N., Fischer, O. M., Streit, S., Hart, S. & Ullrich, A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr. Relat. Cancer 8, 11–31 (2001).
Arteaga, C. L., Moulder, S. L. & Yakes, F. M. HER (erbB) tyrosine kinase inhibitors in the treatment of breast cancer. Semin. Oncol. 29, 4–10 (2002).
Zhang, H. et al. ErbB receptors: from oncogenes to targeted cancer therapies. J. Clin. Invest. 117, 2051–2058 (2007).
Gullick, W. J. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br. Med. Bull. 47, 87–98 (1991).
Mischel, P. S. et al. Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene 22, 2361–2373 (2003).
Andersson, U. et al. Epidermal growth factor receptor family (EGFR, ErbB2–4) in gliomas and meningiomas. Acta Neuropathol. 108, 135–142 (2004).
Pinkas-Kramarski, R. et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 15, 2452–2467 (1996).
Goldman, R., Levy, R. B., Peles, E. & Yarden, Y. Heterodimerization of the erbB-1 and erbB-2 receptors in human breast carcinoma cells: a mechanism for receptor transregulation. Biochemistry 29, 11024–11028 (1990).
Ben-Levy, R., Paterson, H. F., Marshall, C. J. & Yarden, Y. A single autophosphorylation site confers oncogenicity to the Neu/ErbB-2 receptor and enables coupling to the MAP kinase pathway. EMBO J. 13, 3302–3311 (1994).
Klapper, L. N. et al. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene 14, 2099–2109 (1997).
Riethmacher, D. et al. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389, 725–730 (1997).
Houshmandi, S. S., Emnett, R. J., Giovannini, M. & Gutmann, D. H. The neurofibromatosis 2 protein, merlin, regulates glial cell growth in an ErbB2- and Src-dependent manner. Mol. Cell Biol. 29, 1472–1486 (2009).
Ammoun, S., Flaiz, C., Ristic, N., Schuldt, J. & Hanemann, C. O. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 68, 5236–5245 (2008).
Prayson, R. A., Yoder, B. J. & Barnett, G. H. Epidermal growth factor receptor is not amplified in schwannomas. Ann. Diagn. Pathol. 11, 326–329 (2007).
Doherty, J. K., Ongkeko, W., Crawley, B., Andalibi, A. & Ryan, A. F. ErbB and Nrg: potential molecular targets for vestibular schwannoma pharmacotherapy. Otol. Neurotol. 29, 50–57 (2008).
Stonecypher, M. S., Chaudhury, A. R., Byer, S. J. & Carroll, S. L. Neuregulin growth factors and their ErbB receptors form a potential signaling network for schwannoma tumorigenesis. J. Neuropathol. Exp. Neurol. 65, 162–175 (2006).
Wickremesekera, A., Hovens, C. M. & Kaye, A. H. Expression of ErbB-1 and ErbB-2 in meningioma. J. Clin. Neurosci. 17, 1155–1158 (2010).
Curto, M., Cole, B. K., Lallemand, D., Liu, C. H. & McClatchey, A. I. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J. Cell Biol. 177, 893–903 (2007).
Hansen, M. R. & Linthicum, F. H. Jr. Expression of neuregulin and activation of erbB receptors in vestibular schwannomas: possible autocrine loop stimulation. Otol. Neurotol. 25, 155–159 (2004).
Brown, K. D. & Hansen, M. R. Lipid raft localization of ErbB2 in vestibular schwannoma and schwann cells. Otol. Neurotol. 29, 79–85 (2008).
Lallemand, D. et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene 28, 854–865 (2009).
Thaxton, C., Lopera, J., Bott, M. & Fernandez-Valle, C. Neuregulin and laminin stimulate phosphorylation of the NF2 tumor suppressor in Schwann cells by distinct protein kinase A and p21-activated kinase-dependent pathways. Oncogene 27, 2705–2715 (2008).
Ammoun, S. et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 12, 834–843 (2010).
Schaefer, G., Shao, L., Totpal, K. & Akita, R. W. Erlotinib directly inhibits HER2 kinase activation and downstream signaling events in intact cells lacking epidermal growth factor receptor expression. Cancer Res. 67, 1228–1238 (2007).
Clark, J. J. et al. The ErbB inhibitors trastuzumab and erlotinib inhibit growth of vestibular schwannoma xenografts in nude mice: a preliminary study. Otol. Neurotol. 29, 846–853 (2008).
Eccleston, P. A., Funa, K. & Heldin, C. H. Expression of platelet-derived growth factor (PDGF) and PDGF α- and β-receptors in the peripheral nervous system: an analysis of sciatic nerve and dorsal root ganglia. Dev. Biol. 155, 459–470 (1993).
Heldin, C. H. & Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79, 1283–1316 (1999).
Peulve, P., Laquerriere, A., Paresy, M., Hemet, J. & Tadie, M. Establishment of adult rat Schwann cell cultures: effect of b-FGF, α-MSH, NGF, PDGF, and TGF-β on cell cycle. Exp. Cell Res. 214, 543–550 (1994).
Monje, P. V. et al. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia 57, 947–961 (2009).
Maudsley, S. et al. Platelet-derived growth factor receptor association with Na+/H+ exchanger regulatory factor potentiates receptor activity. Mol. Cell Biol. 20, 8352–8363 (2000).
Reczek, D., Berryman, M. & Bretscher, A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 139, 169–179 (1997).
Murthy, A. et al. NHE-RF, a regulatory cofactor for Na+–H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J. Biol. Chem. 273, 1273–1276 (1998).
James, M. F., Beauchamp, R. L., Manchanda, N., Kazlauskas, A. & Ramesh, V. A NHERF binding site links the βPDGFR to the cytoskeleton and regulates cell spreading and migration. J. Cell Sci. 117, 2951–2961 (2004).
Fraenzer, J. T. et al. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int. J. Oncol. 23, 1493–1500 (2003).
Previtali, S. C. et al. Schwann cells synthesize α7β1 integrin which is dispensable for peripheral nerve development and myelination. Mol. Cell Neurosci. 23, 210–218 (2003).
Berti, C., Nodari, A., Wrabetz, L. & Feltri, M. L. Role of integrins in peripheral nerves and hereditary neuropathies. Neuromol. Med. 8, 191–204 (2006).
Hermanson, M. et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 52, 3213–3219 (1992).
Obremski, V. J., Hall, A. M. & Fernandez-Valle, C. Merlin, the neurofibromatosis type 2 gene product, and β1 integrin associate in isolated and differentiating Schwann cells. J. Neurobiol. 37, 487–501 (1998).
Baron, V. & Schwartz, M. Cell adhesion regulates ubiquitin-mediated degradation of the platelet-derived growth factor receptor β. J. Biol. Chem. 275, 39318–39323 (2000).
Miyamoto, S. et al. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131, 791–805 (1995).
Uhrbom, L., Hesselager, G., Ostman, A., Nister, M. & Westermark, B. Dependence of autocrine growth factor stimulation in platelet-derived growth factor-B-induced mouse brain tumor cells. Int. J. Cancer 85, 398–406 (2000).
Lokker, N. A., Sullivan, C. M., Hollenbach, S. J., Israel, M. A. & Giese, N. A. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 62, 3729–3735 (2002).
Servidei, T., Riccardi, A., Sanguinetti, M., Dominici, C. & Riccardi, R. Increased sensitivity to the platelet-derived growth factor (PDGF) receptor inhibitor STI571 in chemoresistant glioma cells is associated with enhanced PDGF-BB-mediated signaling and STI571-induced Akt inactivation. J. Cell. Physiol. 208, 220–228 (2006).
Smith, J. S., Lal, A., Harmon-Smith, M., Bollen, A. W. & McDermott, M. W. Association between absence of epidermal growth factor receptor immunoreactivity and poor prognosis in patients with atypical meningioma. J. Neurosurg. 106, 1034–1040 (2007).
Yang, S. Y. & Xu, G. M. Expression of PDGF and its receptor as well as their relationship to proliferating activity and apoptosis of meningiomas in human meningiomas. J. Clin. Neurosci. 8 (Suppl. 1), 49–53 (2001).
Hilton, D. A., Ristic, N. & Hanemann, C. O. Activation of ERK, AKT and JNK signalling pathways in human schwannomas in situ. Histopathology 55, 744–749 (2009).
Utermark, T., Kaempchen, K. & Hanemann, C. O. Pathological adhesion of primary human schwannoma cells is dependent on altered expression of integrins. Brain Pathol. 13, 352–363 (2003).
Bello, L. et al. αVβ3 and αVβ5 integrin expression in meningiomas. Neurosurgery 47, 1185–1195 (2000).
Wang, Y., Ji, Q. S., Mulvihill, M. & Pachter, J. A. Inhibition of the IGF-I receptor for treatment of cancer. Kinase inhibitors and monoclonal antibodies as alternative approaches. Recent Results Cancer Res. 172, 59–76 (2007).
Clark, J. W., Eder, J. P., Ryan, D., Lathia, C. & Lenz, H. J. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin. Cancer Res. 11, 5472–5480 (2005).
Ammoun, S., Schmid, M. C., Triner, J., Manley, P. & Hanemann, C. O. Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro Oncol. (in press).
Adjei, A. A. et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J. Clin. Oncol. 26, 2139–2146 (2008).
Yeh, T. C. et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin. Cancer Res. 13, 1576–1583 (2007).
Anlar, B., Sullivan, K. A. & Feldman, E. L. Insulin-like growth factor-I and central nervous system development. Horm. Metab. Res. 31, 120–125 (1999).
Russo, V. C., Gluckman, P. D., Feldman, E. L. & Werther, G. A. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr. Rev. 26, 916–943 (2005).
Myers, M. G. Jr et al. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J. Biol. Chem. 269, 28783–28789 (1994).
Mirsky, R. et al. Schwann cells as regulators of nerve development. J. Physiol. Paris 96, 17–24 (2002).
Cheng, H. L. et al. Characterization of insulin-like growth factor-I and its receptor and binding proteins in transected nerves and cultured Schwann cells. J. Neurochem. 66, 525–536 (1996).
Stewart, H. J. et al. Regulation of rat Schwann cell Po expression and DNA synthesis by insulin-like growth factors in vitro. Eur. J. Neurosci. 8, 553–564 (1996).
Russell, J. W., Cheng, H. L. & Golovoy, D. Insulin-like growth factor-I promotes myelination of peripheral sensory axons. J. Neuropathol. Exp. Neurol. 59, 575–584 (2000).
Hofmann, F. & Garcia-Echeverria, C. Blocking the insulin-like growth factor-I receptor as a strategy for targeting cancer. Drug Discov. Today 10, 1041–1047 (2005).
Rodon, J., DeSantos, V., Ferry, R. J. Jr & Kurzrock, R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol. Cancer Ther. 7, 2575–2588 (2008).
Shinkaruk, S., Bayle, M., Lain, G. & Deleris, G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr. Med. Chem. Anticancer Agents 3, 95–117 (2003).
Nussenbaum, F. & Herman, I. M. Tumor angiogenesis: insights and innovations. J. Oncol. 2010, 132641 (2010).
Pilch, H. et al. Hypoxia-stimulated expression of angiogenic growth factors in cervical cancer cells and cervical cancer-derived fibroblasts. Int. J. Gynecol. Cancer 11, 137–142 (2001).
Ellis, L. M. The role of neuropilins in cancer. Mol. Cancer Ther. 5, 1099–1107 (2006).
Plotkin, S. R. et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N. Engl. J. Med. 361, 358–367 (2009).
Caye-Thomasen, P. et al. VEGF and VEGF receptor-1 concentration in vestibular schwannoma homogenates correlates to tumor growth rate. Otol. Neurotol. 26, 98–101 (2005).
Uesaka, T. et al. Expression of VEGF and its receptor genes in intracranial schwannomas. J. Neurooncol. 83, 259–266 (2007).
Zhu, Z. et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia 17, 604–611 (2003).
Xue, Y. et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc. Natl Acad. Sci. USA 105, 18513–18518 (2008).
Zhu, Z. & Witte, L. Inhibition of tumor growth and metastasis by targeting tumor-associated angiogenesis with antagonists to the receptors of vascular endothelial growth factor. Invest. New Drugs 17, 195–212 (1999).
Yagi, Y. et al. Biodistribution of humanized anti-VEGF monoclonal antibody/bevacizumab on peritoneal metastatic models with subcutaneous xenograft of gastric cancer in mice. Cancer Chemother. Pharmacol. 66, 745–753 (2010).
Huynh, H., Chow, P. K. & Soo, K. C. AZD6244 and doxorubicin induce growth suppression and apoptosis in mouse models of hepatocellular carcinoma. Mol. Cancer Ther. 6, 2468–2476 (2007).
Weller, M. & Stupp, R. Approval of new drugs for glioblastoma. Curr. Opin. Neurol. 22, 617–618 (2009).
Rouleau, G. A. et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 363, 515–521 (1993).
Bretscher, A., Edwards, K. & Fehon, R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 (2002).
McClatchey, A. I. & Fehon, R. G. Merlin and the ERM proteins--regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 19, 198–206 (2009).
Li, W. et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4DCAF1 in the nucleus. Cell 140, 477–490 (2010).
Evans, D. G. et al. Consensus recommendations to accelerate clinical trials for neurofibromatosis type 2. Clin. Cancer Res. 15, 5032–5039 (2009).
Leu, T. H. & Maa, M. C. Tyr-863 phosphorylation enhances focal adhesion kinase autophosphorylation at Tyr-397. Oncogene 21, 6992–7000 (2002).
Schlaepfer, D. D. & Hunter, T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem. 272, 13189–13195 (1997).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to researching data for the article, made substantial contributions to the discussions of the content, and contributed equally to writing the article and to reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ammoun, S., Hanemann, C. Emerging therapeutic targets in schwannomas and other merlin-deficient tumors. Nat Rev Neurol 7, 392–399 (2011). https://doi.org/10.1038/nrneurol.2011.82
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2011.82
This article is cited by
-
The genetic landscape and possible therapeutics of neurofibromatosis type 2
Cancer Cell International (2023)
-
Soft and stretchable organic bioelectronics for continuous intraoperative neurophysiological monitoring during microsurgery
Nature Biomedical Engineering (2023)
-
NF2 mutations are associated with resistance to radiation therapy for grade 2 and grade 3 recurrent meningiomas
Journal of Neuro-Oncology (2023)
-
Connective tissue growth factor promotes chemotaxis of preosteoblasts through integrin α5 and Ras during tensile force-induced intramembranous osteogenesis
Scientific Reports (2021)
-
A Novel Imaging Grading Biomarker for Predicting Hearing Loss in Acoustic Neuromas
Clinical Neuroradiology (2021)