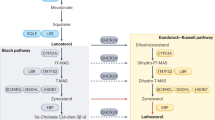
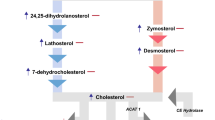
Abstract
The CNS is rich in cholesterol, which is essential for neuronal development and survival, synapse maturation, and optimal synaptic activity. Alterations in brain cholesterol homeostasis are linked to neurodegeneration. Studies have demonstrated that Huntington disease (HD), a progressive and fatal neurodegenerative disorder resulting from polyglutamine expansion in the huntingtin protein, is associated with changes in cellular cholesterol metabolism. Emerging evidence from human and animal studies indicates that attenuated brain sterol synthesis and accumulation of cholesterol in neuronal membranes represent two distinct mechanisms occurring in the presence of mutant huntingtin that influence neuronal survival. Increased knowledge of how changes in intraneuronal cholesterol metabolism influence the pathogenesis of HD will provide insights into the potential application of brain cholesterol regulation as a therapeutic strategy for this devastating disease.
Key Points
-
The brain is a cholesterol-rich organ, and cholesterol has a critical role in brain development, synaptogenesis, and neuronal activity and survival
-
Disturbances in brain cholesterol homeostasis are implicated in neurodegeneration
-
Attenuated plasma cholesterol levels, reduced brain cholesterol synthesis and cholesterol accumulation in neuronal plasma membranes are observed in patients with Huntington disease (HD) and in animal models of the disease
-
Multiple mechanisms are proposed to underlie cholesterol dysregulation in HD, including impaired activation of sterol regulatory element-binding protein, which regulates cholesterogenic gene expression, and reduced brain-derived neurotrophic factor signaling
-
Disturbances in cholesterol homeostasis may influence neuronal survival and susceptibility to excitotoxicity
-
Targeting of cholesterol metabolism represents a potential avenue for treatment to alleviate some neuronal impairments in HD
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
[No authors listed] A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell 72, 971–983 (1993).
Zeron, M. M. et al. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33, 849–860 (2002).
Levine, M. S. et al. Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington's disease. J. Neurosci. Res. 58, 515–532 (1999).
Trushina, E. et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol. Cell Biol. 24, 8195–8209 (2004).
Gauthier, L. R. et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 118, 127–138 (2004).
Graham, R. K. et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell 125, 1179–1191 (2006).
Bjorkhem, I. & Meaney, S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 24, 806–815 (2004).
Dietschy, J. M. & Turley, S. D. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45, 1375–1397 (2004).
Porter, F. D. & Herman, G. E. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52, 6–34 (2011).
Vance, J. E. Lipid imbalance in the neurological disorder, Niemann–Pick C disease. FEBS Lett. 580, 5518–5524 (2006).
Puglielli, L., Tanzi, R. E. & Kovacs, D. M. Alzheimer's disease: the cholesterol connection. Nat. Neurosci. 6, 345–351 (2003).
Wolozin, B. Cholesterol and the biology of Alzheimer's disease. Neuron 41, 7–10 (2004).
Ikonen, E. Mechanisms for cellular cholesterol transport: defects and human disease. Physiol. Rev. 86, 1237–1261 (2006).
Clee, S. M. et al. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 106, 1263–1270 (2000).
Cohen, J. et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37, 161–165 (2005).
Brunham, L. R. et al. β-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13, 340–347 (2007).
Glass, C. K. & Witztum, J. L. Atherosclerosis: the road ahead. Cell 104, 503–516 (2001).
Zlokovic, B. V. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Wilson, J. D. The measurement of the exchangeable pools of cholesterol in the baboon. J. Clin. Invest. 49, 655–665 (1970).
Dehouck, B. et al. A new function for the LDL receptor: transcytosis of LDL across the blood–brain barrier. J. Cell Biol. 138, 877–889 (1997).
Goti, D. et al. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J. Neurochem. 76, 498–508 (2001).
Panzenboeck, U. et al. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood–brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 277, 42781–42789 (2002).
Panzenboeck, U. et al. Regulatory effects of synthetic liver X receptor- and peroxisome-proliferator activated receptor agonists on sterol transport pathways in polarized cerebrovascular endothelial cells. Int. J. Biochem. Cell Biol. 38, 1314–1329 (2006).
Do, T. M. et al. Direct evidence of abca1-mediated efflux of cholesterol at the mouse blood–brain barrier. Mol. Cell Biochem. doi:10.1007/s11010-011-0910-6.
Karasinska, J. M. et al. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 29, 3579–3589 (2009).
Saito, K. et al. Ablation of cholesterol biosynthesis in neural stem cells increases their VEGF expression and angiogenesis but causes neuron apoptosis. Proc. Natl Acad. Sci. USA 106, 8350–8355 (2009).
Pfrieger, F. W. Role of cholesterol in synapse formation and function. Biochim. Biophys. Acta 1610, 271–280 (2003).
Fünfschilling, U., Saher, G., Xiao, L., Mobius, W. & Nave, K. A. Survival of adult neurons lacking cholesterol synthesis in vivo. BMC Neurosci. 8, 1 (2007).
Barres, B. A. & Smith, S. J. Neurobiology. Cholesterol—making or breaking the synapse. Science 294, 1296–1297 (2001).
Mauch, D. H. et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 (2001).
LaDu, M. J. et al. Nascent astrocyte particles differ from lipoproteins in CSF. J. Neurochem. 70, 2070–2081 (1998).
Vance, J. E., Hayashi, H. & Karten, B. Cholesterol homeostasis in neurons and glial cells. Semin. Cell Dev. Biol. 16, 193–212 (2005).
Handelmann, G. E., Boyles, J. K., Weisgraber, K. H., Mahley, R. W. & Pitas, R. E. Effects of apolipoprotein E, β-very low density lipoproteins, and cholesterol on the extension of neurites by rabbit dorsal root ganglion neurons in vitro. J. Lipid Res. 33, 1677–1688 (1992).
de Chaves, E. I., Rusinol, A. E., Vance, D. E., Campenot, R. B. & Vance, J. E. Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J. Biol. Chem. 272, 30766–30773 (1997).
Ignatius, M. J., Shooter, E. M., Pitas, R. E. & Mahley, R. W. Lipoprotein uptake by neuronal growth cones in vitro. Science 236, 959–962 (1987).
Snipes, G. J., McGuire, C. B., Norden, J. J. & Freeman, J. A. Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc. Natl Acad. Sci. USA 83, 1130–1134 (1986).
Cartagena, C. M. et al. Cortical injury increases cholesterol 24S hydroxylase (Cyp46) levels in the rat brain. J. Neurotrauma 25, 1087–1098 (2008).
Fukumoto, H., Deng, A., Irizarry, M. C., Fitzgerald, M. L. & Rebeck, G. W. Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Aβ levels. J. Biol. Chem. 277, 48508–48513 (2002).
Lund, E. G., Guileyardo, J. M. & Russell, D. W. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl Acad. Sci. USA 96, 7238–7243 (1999).
Bjorkhem, I., Lutjohann, D., Breuer, O., Sakinis, A. & Wennmalm, A. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J. Biol. Chem. 272, 30178–30184 (1997).
Lund, E. G. et al. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 278, 22980–22988 (2003).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 (1993).
Jiang, Q. et al. ApoE promotes the proteolytic degradation of Aβ. Neuron 58, 681–693 (2008).
Chu, L. W. et al. A novel intronic polymorphism of ABCA1 gene reveals risk for sporadic Alzheimer's disease in Chinese. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 1007–1013 (2007).
Rodriguez-Rodriguez, E. et al. Association of genetic variants of ABCA1 with Alzheimer's disease risk. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 964–968 (2007).
Hollingworth, P. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 43, 429–435 (2011).
Naj, A. C. et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat. Genet. 43, 436–444 (2011).
Reitz, C. et al. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 67, 1491–1497 (2010).
Hooghwinkel, G. J., Borri, P. F. & Bruyn, G. W. Biochemical studies in Huntington's chorea. II. Composition of blood lipids. Acta Neurol. Scand. 42, 213–220 (1966).
Maltese, W. A. Cholesterol synthesis in cultured skin fibroblasts from patients with Huntington's disease. Biochem. Med. 32, 144–150 (1984).
Leoni, V. et al. Whole body cholesterol metabolism is impaired in Huntington's disease. Neurosci. Lett. 494, 245–249 (2011).
Sipione, S. et al. Early transcriptional profiles in huntingtin-inducible striatal cells by microarray analyses. Hum. Mol. Genet. 11, 1953–1965 (2002).
Valenza, M. et al. Dysfunction of the cholesterol biosynthetic pathway in Huntington's disease. J. Neurosci. 25, 9932–9939 (2005).
del Toro, D. et al. Altered cholesterol homeostasis contributes to enhanced excitotoxicity in Huntington's disease. J. Neurochem. 115, 153–167 (2010).
Leoni, V. et al. Changes in human plasma levels of the brain specific oxysterol 24S-hydroxycholesterol during progression of multiple sclerosis. Neurosci. Lett. 331, 163–166 (2002).
Lutjohann, D. et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J. Lipid Res. 41, 195–198 (2000).
Papassotiropoulos, A. et al. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J. Psychiatr. Res. 36, 27–32 (2002).
Schonknecht, P. et al. Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer's disease compared to healthy controls. Neurosci. Lett. 324, 83–85 (2002).
Leoni, V. et al. Plasma 24S-hydroxycholesterol and caudate MRI in pre-manifest and early Huntington's disease. Brain 131, 2851–2859 (2008).
Valenza, M. et al. Cholesterol biosynthesis pathway is disturbed in YAC128 mice and is modulated by huntingtin mutation. Hum. Mol. Genet. 16, 2187–2198 (2007).
Valenza, M. et al. Progressive dysfunction of the cholesterol biosynthesis pathway in the R6/2 mouse model of Huntington's disease. Neurobiol. Dis. 28, 133–142 (2007).
Valenza, M. et al. Cholesterol defect is marked across multiple rodent models of Huntington's disease and is manifest in astrocytes. J. Neurosci. 30, 10844–10850 (2010).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996).
Van Raamsdonk, J. M. et al. Loss of wild-type huntingtin influences motor dysfunction and survival in the YAC128 mouse model of Huntington disease. Hum. Mol. Genet. 14, 1379–1392 (2005).
Van Raamsdonk, J. M., Warby, S. C. & Hayden, M. R. Selective degeneration in YAC mouse models of Huntington disease. Brain Res. Bull. 72, 124–131 (2007).
Trushina, E. et al. Mutant huntingtin inhibits clathrin-independent endocytosis and causes accumulation of cholesterol in vitro and in vivo. Hum. Mol. Genet. 15, 3578–3591 (2006).
Luthi-Carter, R. et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc. Natl Acad. Sci. USA 107, 7927–7932 (2010).
Reid, P. C. et al. A novel cholesterol stain reveals early neuronal cholesterol accumulation in the Niemann–Pick type C1 mouse brain. J. Lipid Res. 45, 582–591 (2004).
Csallany, A. S., Kindom, S. E., Addis, P. B. & Lee, J. H. HPLC method for quantitation of cholesterol and four of its major oxidation products in muscle and liver tissues. Lipids 24, 645–651 (1989).
Carr, T. P., Andresen, C. J. & Rudel, L. L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 26, 39–42 (1993).
Carlson, S. E. & Goldfarb, S. A sensitive enzymatic method for determination of free and esterified tissue cholesterol. Clin. Chim. Acta 79, 575–582 (1977).
Cattaneo, E., Zuccato, C. & Tartari, M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat. Rev. Neurosci. 6, 919–930 (2005).
Li, S. H. & Li, X. J. Huntingtin–protein interactions and the pathogenesis of Huntington's disease. Trends Genet. 20, 146–154 (2004).
Harjes, P. & Wanker, E. E. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem. Sci. 28, 425–433 (2003).
Sanchez, H. B., Yieh, L. & Osborne, T. F. Cooperation by sterol regulatory element-binding protein and Sp1 in sterol regulation of low density lipoprotein receptor gene. J. Biol. Chem. 270, 1161–1169 (1995).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Rawson, R. B. The SREBP pathway—insights from Insigs and insects. Nat. Rev. Mol. Cell Biol. 4, 631–640 (2003).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Suzuki, S. et al. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J. Neurosci. 27, 6417–6427 (2007).
Zuccato, C. & Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 5, 311–322 (2009).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 (2000).
Parton, R. G. & Richards, A. A. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4, 724–738 (2003).
Murata, M. et al. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl Acad. Sci. USA 92, 10339–10343 (1995).
Thiele, C., Hannah, M. J., Fahrenholz, F. & Huttner, W. B. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat. Cell Biol. 2, 42–49 (2000).
Fu, Y. et al. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J. Biol. Chem. 279, 14140–14146 (2004).
Usdin, M. T., Shelbourne, P. F., Myers, R. M. & Madison, D. V. Impaired synaptic plasticity in mice carrying the Huntington's disease mutation. Hum. Mol. Genet. 8, 839–846 (1999).
Klapstein, G. J. et al. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington's disease transgenic mice. J. Neurophysiol. 86, 2667–2677 (2001).
Ortiz, A. N., Kurth, B. J., Osterhaus, G. L. & Johnson, M. A. Dysregulation of intracellular dopamine stores revealed in the R6/2 mouse striatum. J. Neurochem. 112, 755–761 (2010).
Johnson, M. A. et al. Catecholamine exocytosis is diminished in R6/2 Huntington's disease model mice. J. Neurochem. 103, 2102–2110 (2007).
Chamberlain, L. H., Burgoyne, R. D. & Gould, G. W. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc. Natl Acad. Sci. USA 98, 5619–5624 (2001).
Wojcicka, G., Jamroz-Wisniewska, A., Horoszewicz, K. & Beltowski, J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig. Med. Dosw. (Online) 61, 736–759 (2007).
Joseph, S. B., Castrillo, A., Laffitte, B. A., Mangelsdorf, D. J. & Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213–219 (2003).
Zelcer, N. & Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116, 607–614 (2006).
Hindinger, C. et al. Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J. Neurosci. Res. 84, 1225–1234 (2006).
Lefterov, I. et al. Expression profiling in APP23 mouse brain: inhibition of Aβ amyloidosis and inflammation in response to LXR agonist treatment. Mol. Neurodegener. 2, 20 (2007).
Morales, J. R. et al. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation 118, 1450–1459 (2008).
Repa, J. J. et al. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J. Neurosci. 27, 14470–14480 (2007).
Sironi, L. et al. Treatment with LXR agonists after focal cerebral ischemia prevents brain damage. FEBS Lett. 582, 3396–3400 (2008).
Zelcer, N. et al. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc. Natl Acad. Sci. USA 104, 10601–10606 (2007).
Futter, M. et al. Wild-type but not mutant huntingtin modulates the transcriptional activity of liver X receptors. J. Med. Genet. 46, 438–446 (2009).
Sapp, E. et al. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 60, 161–172 (2001).
Moller, T. Neuroinflammation in Huntington's disease. J. Neural Transm. 117, 1001–1008 (2010).
Allen, J. A., Halverson-Tamboli, R. A. & Rasenick, M. M. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 8, 128–140 (2007).
Li, L., Murphy, T. H., Hayden, M. R. & Raymond, L. A. Enhanced striatal NR2B-containing N-methyl-D-aspartate receptor-mediated synaptic currents in a mouse model of Huntington disease. J. Neurophysiol. 92, 2738–2746 (2004).
Milnerwood, A. J. & Raymond, L. A. Corticostriatal synaptic function in mouse models of Huntington's disease: early effects of huntingtin repeat length and protein load. J. Physiol. 585, 817–831 (2007).
Zeron, M. M. et al. Potentiation of NMDA receptor-mediated excitotoxicity linked with intrinsic apoptotic pathway in YAC transgenic mouse model of Huntington's disease. Mol. Cell Neurosci. 25, 469–479 (2004).
Valencia, A. et al. Mutant huntingtin and glycogen synthase kinase 3-β accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington's disease. J. Neurosci. Res. 88, 179–190 (2010).
Reynolds, C. H., Betts, J. C., Blackstock, W. P., Nebreda, A. R. & Anderton, B. H. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3β. J. Neurochem. 74, 1587–1595 (2000).
Sui, Z., Kovacs, A. D. & Maggirwar, S. B. Recruitment of active glycogen synthase kinase-3 into neuronal lipid rafts. Biochem. Biophys. Res. Commun. 345, 1643–1648 (2006).
Hetman, M., Cavanaugh, J. E., Kimelman, D. & Xia, Z. Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 20, 2567–2574 (2000).
Linseman, D. A. et al. Glycogen synthase kinase-3β phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J. Neurosci. 24, 9993–10002 (2004).
Ross, C. A. & Tabrizi, S. J. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 10, 83–98 (2011).
Okamoto, S. et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med. 15, 1407–1413 (2009).
McGuinness, B. et al. Statins for the treatment of dementia. Cochrane Database of Systematic Reviews, Issue 8. Art. No.: CD007514. doi:10.1002/14651858.CD007514.pub2 (2010).
Ponce, J. et al. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke 39, 1269–1275 (2008).
Butterfield, D. A., Barone, E. & Mancuso, C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol. Res. 64, 180–186 (2011).
Oosterveer, M. H., Grefhorst, A., Groen, A. K. & Kuipers, F. The liver X receptor: control of cellular lipid homeostasis and beyond Implications for drug design. Prog. Lipid Res. 49, 343–352 (2010).
Lund, E. G. et al. Different roles of liver X receptor α and β in lipid metabolism: effects of an α-selective and a dual agonist in mice deficient in each subtype. Biochem. Pharmacol. 71, 453–463 (2006).
Madathil, S. K., Nelson, P. T., Saatman, K. E. & Wilfred, B. R. MicroRNAs in CNS injury: potential roles and therapeutic implications. Bioessays 33, 21–26 (2010).
Rayner, K. J. et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328, 1570–1573 (2010).
Najafi-Shoushtari, S. H. et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328, 1566–1569 (2010).
Acknowledgements
This study was supported by grants from the Canadian Institutes of Health Research (CIHR MOP-106684 and MOP-84438).
Author information
Authors and Affiliations
Contributions
J. M. Karasinska researched data for the article and wrote the text. J. M. Karasinska and M. R. Hayden contributed equally to discussions of content, and review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Karasinska, J., Hayden, M. Cholesterol metabolism in Huntington disease. Nat Rev Neurol 7, 561–572 (2011). https://doi.org/10.1038/nrneurol.2011.132
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2011.132
This article is cited by
-
Potential Use of the Cholesterol Transfer Inhibitor U18666A as a Potent Research Tool for the Study of Cholesterol Mechanisms in Neurodegenerative Disorders
Molecular Neurobiology (2023)
-
Association between serum lipid levels over time and risk of Parkinson’s disease
Scientific Reports (2022)
-
TDP-43 regulates cholesterol biosynthesis by inhibiting sterol regulatory element-binding protein 2
Scientific Reports (2022)
-
Simvastatin impairs hippocampal synaptic plasticity and cognitive function in mice
Molecular Brain (2021)
-
Qki activates Srebp2-mediated cholesterol biosynthesis for maintenance of eye lens transparency
Nature Communications (2021)