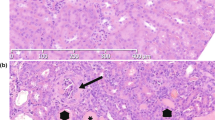
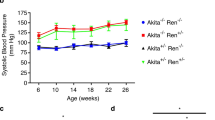
Key Points
-
Numerous renal diseases occur owing to the deposition of a monoclonal immunoglobulin, including multiple myeloma and monoclonal gammopathy of renal significance
-
Understanding the molecular pathogenesis of human immunoglobulin deposition diseases and testing new therapeutic strategies requires relevant animal models, which is a challenge owing to the heterogeneity of these diseases
-
Models based on the injection of purified human immunoglobulins and on tumour grafts that produce the monoclonal immunoglobulin have revealed several early pathogenic events in immunoglobulin deposition and demonstrated the efficacy of innovative therapeutic agents
-
Advances in transgenic techniques have allowed the creation of mouse models that faithfully reproduce the human diseases and have aided in unravelling the pathogenic mechanisms of monoclonal immunoglobulin deposition
-
Animal models are invaluable tools to study the process of deposition and to explore the direct toxicity of monoclonal immunoglobulins in tissues and immunoglobulin-producing plasma cells
Abstract
The renal deposition of monoclonal immunoglobulins can cause severe renal complications in patients with B cell and plasma cell lymphoproliferative disorders. The overproduction of a structurally unique immunoglobulin can contribute to the abnormal propensity of monoclonal immunoglobulins to aggregate and deposit in specific organs. A wide range of renal diseases can occur in multiple myeloma or monoclonal gammopathy of renal significance, including tubular and glomerular disorders with organized or unorganized immunoglobulin deposits. The development of reliable experimental models is challenging owing to the inherent variability of immunoglobulins and the heterogeneity of the pathologies they produce. However, although imperfect, animal models are invaluable tools to understand the molecular pathogenesis of these diseases, and advances in creating genetically modified animals might provide novel approaches to evaluate innovative therapeutic interventions. We discuss the strategies employed to reproduce human monoclonal immunoglobulin-induced kidney lesions in animal models, and we highlight their advantages and shortcomings. We also discuss how these models have affected the management of these deposition diseases and might do so in the future. Finally, we discuss hypotheses that explain some limitations of the various models, and how these models might improve our understanding of other nephropathies without immunoglobulin involvement that have similar pathogenic mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bridoux, F. et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 87, 698–711 (2015).
Merlini, G. & Stone, M. J. Dangerous small B-cell clones. Blood 108, 2520–2530 (2006).
Fermand, J.-P. et al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood 122, 3583–3590 (2013).
Preud'homme, J. L. et al. Monoclonal immunoglobulin deposition disease: a review of immunoglobulin chain alterations. Int. J. Immunopharmacol. 16, 425–431 (1994).
Batuman, V. et al. Myeloma light chains are ligands for cubilin (gp280). Am. J. Physiol. 275, F246–254 (1998).
Christensen, E. I., Birn, H., Storm, T., Weyer, K. & Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology 27, 223–236 (2012).
Hutchison, C. A. et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat. Rev. Nephrol. 8, 43–51 (2012).
Kyle, R. A. et al. Prevalence of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 354, 1362–1369 (2006).
Dispenzieri, A. et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet 375, 1721–1728 (2010).
Leung, N. et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood 120, 4292–4295 (2012).
Sanders, P. W. Mechanisms of light chain injury along the tubular nephron. J. Am. Soc. Nephrol. 23, 1777–1781 (2012).
Maldonado, J. E. et al. Fanconi syndrome in adults. A manifestation of a latent form of myeloma. Am. J. Med. 58, 354–364 (1975).
Messiaen, T. et al. Adult Fanconi syndrome secondary to light chain gammopathy. Clinicopathologic heterogeneity and unusual features in 11 patients. Medicine 79, 135–154 (2000).
Herrera, G. A. Proximal tubulopathies associated with monoclonal light chains: the spectrum of clinicopathologic manifestations and molecular pathogenesis. Arch. Pathol. Lab. Med. 138, 1365–1380 (2014).
Pepys, M. B. Amyloidosis. Annu. Rev. Med. 57, 223–241 (2006).
Desport, E. et al. AL Amyloidosis. Orphanet J. Rare Dis. 7, 54 (2012).
Chiti, F. & Dobson, C. M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006).
Liao, R. et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 104, 1594–1597 (2001).
Brenner, D. A. et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 94, 1008–1010 (2004).
Palladini, G. et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 107, 3854–3858 (2006).
Buxbaum, J. & Gallo, G. Nonamyloidotic monoclonal immunoglobulin deposition disease. Light-chain, heavy-chain, and light- and heavy-chain deposition diseases. Hematol. Oncol. Clin. North Am. 13, 1235–1248 (1999).
Lin, J. et al. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J. Am. Soc. Nephrol. 12, 1482–1492 (2001).
Cohen, C. et al. Randall-type monoclonal immunoglobulin deposition disease: from diagnosis to treatment [French]. Nephrol. Ther. 12, 131–139 (2016).
Aucouturier, P. et al. Brief report: heavy-chain deposition disease. N. Engl. J. Med. 329, 1389–1393 (1993).
Preud'homme, J. L. et al. Monoclonal immunoglobulin deposition disease (Randall type). Relationship with structural abnormalities of immunoglobulin chains. Kidney Int. 46, 965–972 (1994).
Kambham, N. et al. Heavy chain deposition disease: the disease spectrum. Am. J. Kidney Dis. Off. J. Natl Kidney Found. 33, 954–962 (1999).
Bridoux, F. et al. Unravelling the immunopathological mechanisms of heavy chain deposition disease with implications for clinical management. Kidney Int. 91, 423–434 (2017).
Ying, W.-Z., Allen, C. E., Curtis, L. M., Aaron, K. J. & Sanders, P. W. Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J. Clin. Invest. 122, 1777–1785 (2012).
Stevens, F. J. & Argon, Y. Pathogenic light chains and the B-cell repertoire. Immunol. Today 20, 451–457 (1999).
Bellotti, V., Mangione, P. & Merlini, G. Review: immunoglobulin light chain amyloidosis — the archetype of structural and pathogenic variability. J. Struct. Biol. 130, 280–289 (2000).
Blancas-Mejía, L. M. & Ramirez-Alvarado, M. Systemic amyloidoses. Annu. Rev. Biochem. 82, 745–774 (2013).
Davis, D. P. et al. Both the environment and somatic mutations govern the aggregation pathway of pathogenic immunoglobulin light chain. J. Mol. Biol. 313, 1021–1034 (2001).
Wall, J. S. et al. Structural basis of light chain amyloidogenicity: comparison of the thermodynamic properties, fibrillogenic potential and tertiary structural features of four Vlambda6 proteins. J. Mol. Recognit. 17, 323–331 (2004).
Poshusta, T. L. et al. Mutations in specific structural regions of immunoglobulin light chains are associated with free light chain levels in patients with AL amyloidosis. PLoS ONE 4, e5169 (2009).
Hernández-Santoyo, A. et al. A single mutation at the sheet switch region results in conformational changes favoring lambda6 light-chain fibrillogenesis. J. Mol. Biol. 396, 280–292 (2010).
Kobayashi, Y. et al. Decreased amyloidogenicity caused by mutational modulation of surface properties of the immunoglobulin light chain BRE variable domain. Biochemistry 53, 5162–5173 (2014).
Comenzo, R. L., Zhang, Y., Martinez, C., Osman, K. & Herrera, G. A. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood 98, 714–720 (2001).
Perfetti, V. et al. Analysis of V(lambda)-J(lambda) expression in plasma cells from primary (AL) amyloidosis and normal bone marrow identifies 3r (lambdaIII) as a new amyloid-associated germline gene segment. Blood 100, 948–953 (2002).
Abraham, R. S. et al. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood 101, 3801–3808 (2003).
Perfetti, V. et al. The repertoire of λ light chains causing predominant amyloid heart involvement and identification of a preferentially involved germline gene, IGLV1-44. Blood 119, 144–150 (2012).
Rocca, A. et al. Primary structure of a variable region of the V kappa I subgroup (ISE) in light chain deposition disease. Clin. Exp. Immunol. 91, 506–509 (1993).
Denoroy, L., Déret, S. & Aucouturier, P. Overrepresentation of the V kappa IV subgroup in light chain deposition disease. Immunol. Lett. 42, 63–66 (1994).
Decourt, C., Cogné, M. & Rocca, A. Structural peculiarities of a truncated V kappa III immunoglobulin light chain in myeloma with light chain deposition disease. Clin. Exp. Immunol. 106, 357–361 (1996).
Kaplan, B., Livneh, A. & Gallo, G. Charge differences between in vivo deposits in immunoglobulin light chain amyloidosis and non-amyloid light chain deposition disease. Br. J. Haematol. 136, 723–728 (2007).
Isobe, T., Kametani, F. & Shinoda, T. V-Domain deposition of lambda Bence Jones protein in the renal tubular epithelial cells in a patient with the adult Fanconi syndrome with myeloma. Amyloid 5, 117–120 (1998).
Bridoux, F. et al. Fanconi's syndrome induced by a monoclonal Vkappa3 light chain in Waldenstrom's macroglobulinemia. Am. J. Kidney Dis. 45, 749–757 (2005).
Aucouturier, P. et al. Monoclonal Ig L chain and L chain V domain fragment crystallization in myeloma-associated Fanconi's syndrome. J. Immunol. 150, 3561–3568 (1993).
Leboulleux, M. et al. Protease resistance and binding of Ig light chains in myeloma-associated tubulopathies. Kidney Int. 48, 72–79 (1995).
Decourt, C. et al. Mutational analysis in murine models for myeloma-associated Fanconi's syndrome or cast myeloma nephropathy. Blood 94, 3559–3566 (1999).
Luciani, A. et al. Impaired lysosomal function underlies monoclonal light chain-associated renal Fanconi syndrome. J. Am. Soc. Nephrol. 27, 2049–2061 (2016).
Nasr, S. H. et al. The diagnosis and characteristics of renal heavy-chain and heavy/light-chain amyloidosis and their comparison with renal light-chain amyloidosis. Kidney Int. 83, 463–470 (2013).
Khamlichi, A. A., Aucouturier, P., Preud'homme, J. L. & Cogné, M. Structure of abnormal heavy chains in human heavy-chain-deposition disease. Eur. J. Biochem. 229, 54–60 (1995).
Hendershot, L., Bole, D., Köhler, G. & Kearney, J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J. Cell Biol. 104, 761–767 (1987).
Cogné, M., Silvain, C., Khamlichi, A. A. & Preud'homme, J. L. Structurally abnormal immunoglobulins in human immunoproliferative disorders. Blood 79, 2181–2195 (1992).
Sanders, P. W., Herrera, G. A. & Galla, J. H. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 32, 851–861 (1987).
Sanders, P. W., Herrera, G. A., Chen, A., Booker, B. B. & Galla, J. H. Differential nephrotoxicity of low molecular weight proteins including Bence Jones proteins in the perfused rat nephron in vivo. J. Clin. Invest. 82, 2086–2096 (1988).
Solomon, A., Weiss, D. T. & Kattine, A. A. Nephrotoxic potential of Bence Jones proteins. N. Engl. J. Med. 324, 1845–1851 (1991).
Solomon, A., Weiss, D. T. & Pepys, M. B. Induction in mice of human light-chain-associated amyloidosis. Am. J. Pathol. 140, 629–637 (1992).
Khan, A.-M. et al. Myeloma light chain-induced renal injury in mice. Nephron Exp. Nephrol. 116, e32–e41 (2010).
Khamlichi, A. A. et al. Role of light chain variable region in myeloma with light chain deposition disease: evidence from an experimental model. Blood 86, 3655–3659 (1995).
Rognoni, P. et al. A strategy for synthesis of pathogenic human immunoglobulin free light chains in E. coli. PLoS ONE 8, e76022 (2013).
Teng, J., Turbat-Herrera, E. A. & Herrera, G. A. An animal model of glomerular light-chain-associated amyloidogenesis depicts the crucial role of lysosomes. Kidney Int. 86, 738–746 (2014).
Koss, M. N., Pirani, C. L. & Osserman, E. F. Experimental Bence Jones cast nephropathy. Lab. Invest. 34, 579–591 (1976).
Mishra, S. et al. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am. J. Physiol. Heart Circ. Physiol. 305, H95–H103 (2013).
Diomede, L. et al. A Caenorhabditis elegans-based assay recognizes immunoglobulin light chains causing heart amyloidosis. Blood 123, 3543–3552 (2014).
Zhou, P., Ma, X., Iyer, L., Chaulagain, C. & Comenzo, R. L. One siRNA pool targeting the λ constant region stops λ light-chain production and causes terminal endoplasmic reticulum stress. Blood 123, 3440–3451 (2014).
Hovey, B. M. et al. Preclinical development of siRNA therapeutics for AL amyloidosis. Gene Ther. 18, 1150–1156 (2011).
Chauveau, C., Decourt, C. & Cogné, M. Insertion of the IgH locus 3′ regulatory palindrome in expression vectors warrants sure and efficient expression in stable B cell transfectants. Gene 222, 279–285 (1998).
Nuvolone, M. et al. Regulated expression of amyloidogenic immunoglobulin light chains in mice. Amyloid 24, 52–53 (2017).
Ward, J. E. et al. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood 118, 6610–6617 (2011).
Sirac, C. et al. Role of the monoclonal kappa chain V domain and reversibility of renal damage in a transgenic model of acquired Fanconi syndrome. Blood 108, 536–543 (2006).
Sirac, C. et al. Strategies to model AL amyloidosis in mice. Amyloid 18 (Suppl. 1), 40–42 (2011).
Bonaud, A. et al. A mouse model recapitulating human monoclonal heavy chain deposition disease evidences the relevance of proteasome inhibitor therapy. Blood 126, 757–765 (2015).
Casola, S. et al. B cell receptor signal strength determines B cell fate. Nat. Immunol. 5, 317–327 (2004).
Lechouane, F. et al. B-Cell receptor signal strength influences terminal differentiation. Eur. J. Immunol. 43, 619–628 (2013).
Hrncic, R. et al. Antibody-mediated resolution of light chain-associated amyloid deposits. Am. J. Pathol. 157, 1239–1246 (2000).
Solomon, A., Weiss, D. T. & Wall, J. S. Therapeutic potential of chimeric amyloid-reactive monoclonal antibody 11-1F4. Clin. Cancer Res. 9, 3831S–3838S (2003).
Wall, J. S. et al. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PLoS ONE 7, e52686 (2012).
Gertz, M. A. et al. First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction. J. Clin. Oncol. 34, 1097–1103 (2016).
Nightingale, C. H. & Mouravieff, M. Reliable and simple method of intravenous injection into the laboratory rat. J. Pharm. Sci. 62, 860–861 (1973).
Teng, J. et al. Different types of glomerulopathic light chains interact with mesangial cells using a common receptor but exhibit different intracellular trafficking patterns. Lab. Invest. 84, 440–451 (2004).
Teng, J., Turbat-Herrera, E. A. & Herrera, G. A. Extrusion of amyloid fibrils to the extracellular space in experimental mesangial AL-amyloidosis: transmission and scanning electron microscopy studies and correlation with renal biopsy observations. Ultrastruct. Pathol. 38, 104–115 (2014).
Kluve-Beckerman, B., Manaloor, J. J. & Liepnieks, J. J. A pulse-chase study tracking the conversion of macrophage-endocytosed serum amyloid A into extracellular amyloid. Arthritis Rheum. 46, 1905–1913 (2002).
Lundmark, K., Vahdat Shariatpanahi, A. & Westermark, G. T. Depletion of spleen macrophages delays AA amyloid development: a study performed in the rapid mouse model of AA amyloidosis. PLoS ONE 8, e79104 (2013).
Kennel, S. J. et al. Phagocyte depletion inhibits AA amyloid accumulation in AEF-induced huIL-6 transgenic mice. Amyloid 21, 45–53 (2014).
Arendt, B. K. et al. Biologic and genetic characterization of the novel amyloidogenic lambda light chain-secreting human cell lines, ALMC-1 and ALMC-2. Blood 112, 1931–1941 (2008).
Buxbaum, J. N. Animal models of human amyloidoses: are transgenic mice worth the time and trouble? FEBS Lett. 583, 2663–2673 (2009).
Wechalekar, A. D. & Whelan, C. Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 7, e546 (2017).
Rokita, H., Shirahama, T., Cohen, A. S. & Sipe, J. D. Serum amyloid A gene expression and AA amyloid formation in A/J and SJL/J mice. Br. J. Exp. Pathol. 70, 327–335 (1989).
Takeda, T. et al. A novel murine model of aging, Senescence-Accelerated Mouse (SAM). Arch. Gerontol. Geriatr. 19, 185–192 (1994).
Ge, F. et al. Amyloidosis in transgenic mice expressing murine amyloidogenic apolipoprotein A-II (Apoa2c). Lab. Invest. 87, 633–643 (2007).
Kohno, K. et al. Analysis of amyloid deposition in a transgenic mouse model of homozygous familial amyloidotic polyneuropathy. Am. J. Pathol. 150, 1497–1508 (1997).
Simons, J. P. et al. Pathogenetic mechanisms of amyloid A amyloidosis. Proc. Natl Acad. Sci. USA 110, 16115–16120 (2013).
Chesi, M. et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell 13, 167–180 (2008).
Carrasco, D. R. et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 11, 349–360 (2007).
Hamouda, M.-A. et al. BCL-B (BCL2L10) is overexpressed in patients suffering from multiple myeloma (MM) and drives an MM-like disease in transgenic mice. J. Exp. Med. 213, 1705–1722 (2016).
Shi, J. et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc. Natl Acad. Sci. USA 107, 4188–4193 (2010).
Guan, J. et al. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol. Med. 6, 1493–1507 (2014).
Shin, J. T. et al. Overexpression of human amyloidogenic light chains causes heart failure in embryonic zebrafish: a preliminary report. Amyloid 19, 191–196 (2012).
Diomede, L. et al. Cardiac light chain amyloidosis: the role of metal ions in oxidative stress and mitochondrial damage. Antioxid. Redox Signal. 27, 567–582 (2017).
Herrera, G. A., Turbat-Herrera, E. A. & Teng, J. Animal models of light chain deposition disease provide a better understanding of nodular glomerulosclerosis. Nephron 132, 119–136 (2016).
Ronco, P., Plaisier, E. & Aucouturier, P. Monoclonal immunoglobulin light and heavy chain deposition diseases: molecular models of common renal diseases. Contrib. Nephrol. 169, 221–231 (2011).
Zheng, F., Striker, G. E., Esposito, C., Lupia, E. & Striker, L. J. Strain differences rather than hyperglycemia determine the severity of glomerulosclerosis in mice. Kidney Int. 54, 1999–2007 (1998).
Ma, L.-J. & Fogo, A. B. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int. 64, 350–355 (2003).
Oliva, L. et al. The amyloidogenic light chain is a stressor that sensitizes plasma cells to proteasome inhibitor toxicity. Blood 129, 2132–2142 (2017).
Meister, S. et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 67, 1783–1792 (2007).
Nasr, S. H. et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin. J. Am. Soc. Nephrol. 7, 231–239 (2012).
Cohen, C. et al. Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int. 88, 1135–1143 (2015).
Venner, C. P. et al. A matched comparison of cyclophosphamide, bortezomib and dexamethasone (CVD) versus risk-adapted cyclophosphamide, thalidomide and dexamethasone (CTD) in AL amyloidosis. Leukemia 28, 2304–2310 (2014).
Palladini, G. et al. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia 28, 2311–2316 (2014).
Jaccard, A. et al. Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naïve patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III). Haematologica 99, 1479–1485 (2014).
Kastritis, E. et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J. Clin. Oncol. 28, 1031–1037 (2010).
Batuman, V., Sastrasinh, M. & Sastrasinh, S. Light chain effects on alanine and glucose uptake by renal brush border membranes. Kidney Int. 30, 662–665 (1986).
Batuman, V., Guan, S., O'Donovan, R. & Puschett, J. B. Effect of myeloma light chains on phosphate and glucose transport in renal proximal tubule cells. Ren. Physiol. Biochem. 17, 294–300 (1994).
Pote, A., Zwizinski, C., Simon, E. E., Meleg-Smith, S. & Batuman, V. Cytotoxicity of myeloma light chains in cultured human kidney proximal tubule cells. Am. J. Kidney Dis. 36, 735–744 (2000).
Sengul, S., Zwizinski, C. & Batuman, V. Role of MAPK pathways in light chain-induced cytokine production in human proximal tubule cells. Am. J. Physiol. Renal Physiol. 284, F1245–F1254 (2003).
Wang, P.-X. & Sanders, P. W. Immunoglobulin light chains generate hydrogen peroxide. J. Am. Soc. Nephrol. 18, 1239–1245 (2007).
Li, M., Balamuthusamy, S., Simon, E. E. & Batuman, V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am. J. Physiol. Renal Physiol. 295, F82–F90 (2008).
Ying, W.-Z., Wang, P.-X., Aaron, K. J., Basnayake, K. & Sanders, P. W. Immunoglobulin light chains activate nuclear factor-κB in renal epithelial cells through a Src-dependent mechanism. Blood 117, 1301–1307 (2011).
Sirac, C. et al. Toward understanding renal Fanconi syndrome: step by step advances through experimental models. Contrib. Nephrol. 169, 247–261 (2011).
Raggi, C. et al. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum. Mol. Genet. 23, 2266–2278 (2014).
Aufman, J. & Herrera, G. A. Circulating monoclonal light chains and acute kidney injury: the role of the renal biopsy with emphasis on ultrastructural evaluation in assessing and understanding renal injury. Ultrastruct. Pathol. 39, 159–168 (2015).
Weiss, J. H. et al. Pathophysiology of acute Bence-Jones protein nephrotoxicity in the rat. Kidney Int. 20, 198–210 (1981).
Sanders, P. W. & Booker, B. B. Pathobiology of cast nephropathy from human Bence Jones proteins. J. Clin. Invest. 89, 630–639 (1992).
Huang, Z. Q., Kirk, K. A., Connelly, K. G. & Sanders, P. W. Bence Jones proteins bind to a common peptide segment of Tamm-Horsfall glycoprotein to promote heterotypic aggregation. J. Clin. Invest. 92, 2975–2983 (1993).
Huang, Z. Q. & Sanders, P. W. Biochemical interaction between Tamm-Horsfall glycoprotein and Ig light chains in the pathogenesis of cast nephropathy. Lab. Invest. 73, 810–817 (1995).
Huang, Z. Q. & Sanders, P. W. Localization of a single binding site for immunoglobulin light chains on human Tamm-Horsfall glycoprotein. J. Clin. Invest. 99, 732–736 (1997).
Ying, W. Z. & Sanders, P. W. Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein. Am. J. Pathol. 158, 1859–1866 (2001).
Korbet, S. M. & Schwartz, M. M. Multiple myeloma. J. Am. Soc. Nephrol. 17, 2533–2545 (2006).
Drayson, M. et al. Effects of paraprotein heavy and light chain types and free light chain load on survival in myeloma: an analysis of patients receiving conventional-dose chemotherapy in Medical Research Council UK multiple myeloma trials. Blood 108, 2013–2019 (2006).
Richards, D. B. et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N. Engl. J. Med. 373, 1106–1114 (2015).
Blancas-Mejía, L. M., Misra, P. & Ramirez-Alvarado, M. Differences in protein concentration dependence for nucleation and elongation in light chain amyloid formation. Biochemistry 56, 757–766 (2017).
Becker, G. J. & Hewitson, T. D. Animal models of chronic kidney disease: useful but not perfect. Nephrol. Dial. Transplant. 28, 2432–2438 (2013).
Srour, N. et al. A plasma cell differentiation quality control ablates B cell clones with biallelic Ig rearrangements and truncated Ig production. J. Exp. Med. 213, 109–122 (2016).
Acknowledgements
The authors thank the members of the International Kidney and Monoclonal Gammopathy Research Group for their intellectual support in this project. C.S. thanks C. Carrion, A. Rinsant, S. Kaaki, N. Quellard, J.M. Goujon, A. Jaccard and D. Lavergne for their technical and intellectual support. C.S. is supported by a grant from Fondation Française pour le Recheche contre le Myelome et les Gammapathies Monoclonales. G.A.H. is supported by a grant from the Amyloidosis Foundation. P.W.S. is supported by grants from the Office of Research and Development, Medical Research Service, US Department of Veterans Affairs (I01 CX001326) and the US National Institutes of Health George M. O'Brien Kidney and Urological Research Centers Program (P30 DK079337). M.V.A. is funded by a fellowship from region Nouvelle Aquitaine. S.B. is supported by the French Ministry of Research 'Plan maladies rares'.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article and writing, reviewing and editing the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Immunoglobulin-related amyloidosis
-
Accumulation of abnormal immunoglobulin fragments that form fibrils and deposit in organs and tissues, causing their dysfunction.
- Monoclonal immunoglobulin deposition disease
-
(MIDD). Accumulation of abnormal immunoglobulin fragments that form granular deposits in organs and tissues, mainly the kidneys, causing their dysfunction.
- Myeloma cast nephropathy
-
(MCN). Acute kidney disease that occurs in multiple myeloma and is characterized by the obstruction of distal tubules by casts composed of a monoclonal immunoglobulin light chain.
- Light chain-induced Fanconi syndrome
-
Generalized dysfunction of reabsorption in proximal tubules due to the intracellular accumulation of a monoclonal immunoglobulin light chain.
Rights and permissions
About this article
Cite this article
Sirac, C., Herrera, G., Sanders, P. et al. Animal models of monoclonal immunoglobulin-related renal diseases. Nat Rev Nephrol 14, 246–264 (2018). https://doi.org/10.1038/nrneph.2018.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2018.8
This article is cited by
-
Glomerulonephritis: immunopathogenesis and immunotherapy
Nature Reviews Immunology (2023)
-
Crystalline deposits in the cornea and various areas of the kidney as symptoms of an underlying monoclonal gammopathy: a case report
BMC Nephrology (2021)
-
Kidney injury and disease in patients with haematological malignancies
Nature Reviews Nephrology (2021)
-
The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group
Nature Reviews Nephrology (2019)