Key Points
-
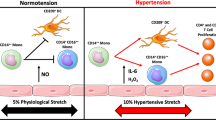
Angiotensin-converting enzyme (ACE) expression by myeloid cells is increased in response to infection.
-
Forced ACE overexpression in mouse macrophages increases their ability to respond to infection and some tumour models, which is in part mediated by increased levels of nitric oxide, superoxide (O2−) and pro-inflammatory cytokines.
-
Forced ACE overexpression in neutrophils increases their response to infection by increasing NADPH oxidase-dependent production of O2−.
-
The effects of ACE overexpression are not mediated by angiotensin II, any other angiotensin peptides or the type 1 angiotensin II receptor but instead by unknown ACE substrate(s) or product(s).
-
No known immunological framework can currently explain the effects of ACE overexpression, but an as yet unrecognized pathway capable of stimulating myeloid function beyond levels achievable by wild-type cells must exist.
Abstract
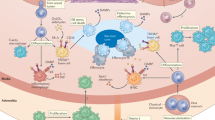
Angiotensin-converting enzyme (ACE) — a zinc-dependent dicarboxypeptidase with two catalytic domains — plays a major part in blood pressure regulation by converting angiotensin I to angiotensin II. However, ACE cleaves many peptides besides angiotensin I and thereby affects diverse physiological functions, including renal development and male reproduction. In addition, ACE has a role in both innate and adaptive responses by modulating macrophage and neutrophil function — effects that are magnified when these cells overexpress ACE. Macrophages that overexpress ACE are more effective against tumours and infections. Neutrophils that overexpress ACE have an increased production of superoxide, which increases their ability to kill bacteria. These effects are due to increased ACE activity but are independent of angiotensin II. ACE also affects the display of major histocompatibility complex (MHC) class I and MHC class II peptides, potentially by enzymatically trimming these peptides. Understanding how ACE expression and activity affect myeloid cells may hold great promise for therapeutic manipulation, including the treatment of both infection and malignancy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Skeggs, L. T. Jr Discovery of the two angiotensin peptides and the angiotensin converting enzyme. Hypertension 21, 259–260 (1993).
Skeggs, L. T. Jr., Marsh, W. H., Kahn, J. R. & Shumway, N. P. The existence of two forms of hypertensin. J. Exp. Med. 99, 275–282 (1954).
Metzger, R. et al. Heterogeneous distribution of angiotensin I-converting enzyme (CD143) in the human and rat vascular systems: vessel, organ and species specificity. Microvasc. Res. 81, 206–215 (2011).
Bernstein, K. E. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 65, 1–46 (2012).
Rigat, B. et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 86, 1343–1346 (1990).
Dux, S. et al. Serum angiotensin converting enzyme activity in normal adults and patients with different types of hypertension. Isr. J. Med. Sci. 20, 1138–1142 (1984).
Bénéteau-Burnat, B., Baudin, B., Morgant, G., Baumann, F. C. & Giboudeau, J. Serum angiotensin-converting enzyme in healthy and sarcoidotic children: comparison with the reference interval for adults. J. Clin. Chem. 36, 344–346 (1990).
Cushman, D. W. & Ondetti, M. A. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 17, 589–592 (1991).
Mentz, R. J. et al. The past, present and future of renin-angiotensin aldosterone system inhibition. Int. J. Cardiol. 167, 1677–1687 (2013).
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N. Engl. J. Med. 316, 1429–1435 (1987).
The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 325, 293–302 (1991).
Lieberman, J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am. J. Med. 59, 365–372 (1975).
Silverstein, E., Pertschuk, L. P. & Friedland, J. Immunofluorescent localization of angiotensin converting enzyme in epithelioid and giant cells of sarcoidosis granulomas. Proc. Natl Acad. Sci. USA 76, 6646–6648 (1979).
Smithies, O., Kim. H. S., Takahashi, N. & Edgell, M. H. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 58, 2265–2280 (2000).
Kim, S. & Iwao, H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 52, 11–34 (2000).
Krege, J. H. et al. Male-female differences in fertility and blood pressure in ACE deficient mice. Nature 375, 146–148 (1995).
Esther, C. R. Jr et al. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab. Invest. 74, 953–965 (1996).
Gribouval, O. et al. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum. Mutat. 33, 316–326 (2012).
Ertoy, D. & Bernstein, K. E. in Drugs, Enzymes and Receptors of the Renin-Angiotensin System: Celebrating a Century of Discovery (eds Husain, A. & Graham, R.) 205–223 (Harwood Academic Publishers, The Netherlands, 2000).
Kim, H. S. et al. Genetic control of blood pressure and the angiotensinogen locus. Proc. Natl Acad. Sci. USA 92, 2735–2739 (1995).
Yanai, K. et al. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J. Biol. Chem. 275, 5–8 (2000).
Tsuchida, S. et al. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J. Clin. Invest. 101, 755–760 (1998).
Miyazaki, Y. et al. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J. Clin. Invest. 102, 1489–1497 (1998).
Fuchs, S. et al. Male fertility is dependent on dipeptidase activity of testis ACE. Nat. Med. 11, 1140–1142 (2005).
Li, L. J. et al. Human sperm devoid of germinal angiotensin-converting enzyme is responsible for total fertilization failure and lower fertilization rates by conventional in vitro fertilization. Biol. Reprod. 90 125, 1–7 (2014).
Hagaman, J. R. et al. Angiotensin-converting enzyme and male fertility. Proc. Natl Acad. Sci. USA 95, 2552–2557 (1998).
Ramaraj, P., Kessler, S. P., Colmenares, C. & Sen, G. C. Selective restoration of male fertility in mice lacking angiotensin-converting enzymes by sperm-specific expression of the testicular isozyme. J. Clin. Invest. 102, 371–378 (1998).
Marchesi, C., Paradis, P. & Schiffrin, E. L. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 29, 367–374 (2008).
Benigni, A., Cassis, P. & Remuzzi, G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol. Med. 2, 247–257 (2010).
Montezano, A. C., Nguyen Dinh Cat, A., Rios, F. J. & Touyz, R. M. Angiotensin II and vascular injury. Curr. Hypertens. Rep. 16, 431 (2014).
Mateo, T. et al. Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J. Immunol. 176, 5577–5586 (2006).
Nathan, C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 111, 769–778 (2003).
Torres, M. & Forman, H. J. Redox signaling and the MAP kinase pathways. Biofactors 17, 287–296 (2003).
Biancardi, V. C., Bomfim, G. F., Reis, W. L., Al-Gassimi, S. & Nunes, K. P. The interplay between Angiotensin II, TLR4 and hypertension. Pharmacol. Res. 120, 88–96 (2017).
Meng, Y., Chen, C., Liu, Y., Tian, C. & Li, H. H. Angiotensin II regulates dendritic cells through activation of NF-κB /p65, ERK1/2 and STAT1 pathways. Cell Physiol. Biochem. 42, 1550–1558 (2017).
Hoch, N. E. et al. Regulation of T cell function by endogenously produced angiotensin II. Am. J. Physiol. Reful Integr. Comp. Physiol. 296, R208–R216 (2009).
Platten, M. et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc. Natl Acad. Sci. USA 106, 14948–14953 (2009).
Lanz, T. V. et al. Angiotensin II sustains brain inflammation in mice via TGF-beta. J. Clin. Invest. 120, 2782–2794 (2010).
Crowley, S., D. & Rudemiller, N. P. Immunologic Effects of the Renin-Angiotensin System. J. Am. Soc. Nephrol. 28, 1350–1361 (2017).
Crowley, S. D. et al. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 55, 99–108 (2010).
Zhang, J. D. et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ. Res. 110, 1604–1617 (2012).
Zhang, J. D. et al. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J. Clin. Invest. 124, 2198–2203 (2014).
Ma, L. J. et al. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am. J. Physiol. Renal Physiol. 300, F1203–F1213 (2011).
Brice, E. A., Friedlander, W., Bateman, E. D. & Kirsch, R. E. Serum angiotensin-converting enzyme activity, concentration, and specific activity in granulomatous interstitial lung disease, tuberculosis, and COPD. Chest 107, 706–710 (1995).
Lieberman, J. & Rea, T. H. Serum angiotensin converting enzyme in leprosy and coccidioidomycosis. Ann. Intern. Med. 87, 422–425 (1977).
Olle, E. W. et al. Screening of serum samples from Wegener's granulomatosis patients using antibody microarrays. Proteom. Clin. Appl. 1, 1212–1220 (2007).
Weinstock, J. V. Production of neuropeptides by inflammatory cells within the granulomas of murine schistosomiasis mansoni. Eur. J. Clin. Invest. 21, 145–153 (1991).
Williams, G. T. & Williams, W. J. Granulomatous inflammation - a review. J. Clin. Pathol. 36, 723–733 (1983).
Nathan, C. Macrophages' choice: take it in or keep it out. Immunity 45, 710–711 (2016).
Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 12, 352–366 (2012).
Sylvius, F. Opera Medica (A. Wolfgang, 1679).
Bean, A. G. et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162, 3504–3511 (1999).
Roach, D. R. et al. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168, 4620–4627 (2002).
Bouley, D. M., Ghori, N., Mercer, K. L., Falkow, S. & Ramakrishnan, L. Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas. Infect. Immun. 69, 7820–7831 (2001).
Helming, L. & Gordon, S. The molecular basis of macrophage fusion. Immunobiology 212, 785–793 (2007).
Cronan et al. Macrophage epithelial reprogramming underlies mycobacterial granuloma formation and promotes infection. Immunity 45, 861–876 (2016).
Shen, X. Z. et al. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am. J. Pathol. 170, 2122–2134 (2007).
Shen, X. Z. et al. Myeloid expression of angiotensin-converting enzyme facilitates myeloid maturation and inhibits the development of myeloid-derived suppressor cells. Lab Invest. 94, 536–544 (2014).
Okwan-Duodu, D. et al. Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 285, 39051–39060 (2010).
Pamer, E. & Cresswell, P. Mechanism of MHC class I− resticted antigen processing. Annu. Rev. Immunol. 16, 323–358 (1998).
Purcell, A. W. & Elliott, T. Molecular machinations of the MHC-I peptide loading complex. Curr. Opin. Immunol. 20, 75–81 (2008).
Shen, X. Z., Lukacher, A. E., Billet, S., Williams, I. R. & Bernstein, K. E. Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide presentation by modifying C termini of peptide precursors. J. Biol. Chem. 283, 9957–9965 (2008).
Shen, X. Z. et al. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat. Immunol. 12, 1078–1085 (2011).
Gonzalez-Villalobos, R. A. et al. Rediscovering ACE: novel insights into the many roles of the angiotensin converting enzyme. J. Mol. Med. 91, 1143–1154 (2013).
Zhao, T., Bernstein, K. E., Fang, J. & Shen, X. Z. Angiotensin-converting enzyme affects the presentation of MHC class II antigens. Lab. Invest. 97, 764–771 (2017).
de Oliveira, S., Rosowski, E. E. & Huttenlocher, A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat. Rev. Immunol. 16, 378–391 (2016).
Guerra, F. E., Borgogna, T. R., Patel, D. M., Sward, E. W. & Voyich, J. M. Epic immune battles of history: neutrophils versus Staphylococcus aureus. Front. Cell. Infect. Microbiol. 7, 286 (2017).
Khan, Z. et al. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood 130, 328–339 (2017).
Winterbourn, C. C., Kettle, A. J. & Hampton, M. B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 85, 765–792 (2016).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 (2010).
Remijsen, Q. et al. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18, 581–588 (2011).
Branzk, N. et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025 (2014).
Branzk, N. & Papayannopoulos, V. Molecular mechanisms regulating NETosis in infection and disease. Semin. Immunopathol. 35, 513–530 (2013).
Pouwels, K. B., Visser, S. T. & Hak, E. Effect of pravastatin and fosinopril on recurrent urinary tract infections. J. Antimicrob. Chemother. 68, 708–714 (2013).
Pouwels, K. B., Bos, J. H. & Hak, E. ACE inhibitors and urinary tract infections. Epidemiology 25, 466–467 (2014).
Dial, S., Nessim, S. J., Kezouh, A., Benisty, J. & Suissa, S. Antihypertensive agents acting on the renin-angiotensin system and the risk of sepsis. Br. J. Clin. Pharmacol. 78, 1151–1158 (2014).
Mortensen, E. M., Restrepo, M. I., Anzueto, A. & Pugh, J. The impact of prior outpatient ACE inhibitor use on 30-day mortality for patients hospitalized with community-acquired pneumonia. BMC Pulm. Med. 5, 12 (2005).
Mortensen, E. M. et al. Impact of statins and angiotensin converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur. Respir. J. 31, 611–617 (2008).
Sobczuk, P., Szczylik, C., Porta, C. & Czarnecka, A. M. Renin angiotensin system deregulation as renal cancer risk factor. Oncol. Lett. 14, 5059–5068 (2017).
Pinter, M. & Jain, R. K. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl Med. 9, eaan5616 (2017).
Steinman, L. Development of therapies for autoimmune disease at Stanford: a tale of multiple shots and one goal. Immunol. Res. 58, 307–314 (2014).
Lühder, F., Lee, D. H., Gold, R., Stegbauer, J. & Linker, R. A. Small but powerful: short peptide hormones and their role in autoimmune inflammation. J. Neuroimmunol. 217, 1–7 (2009).
Fahmy Wahba, M. G., Shehata Messiha, B. A. & Abo-Saif, A. A. Ramipril and haloperidol as promising approaches in managing rheumatoid arthritis in rats. Eur. J. Pharmacol. 765, 307–315 (2015).
Sagawa, K., Nagatani, K., Komagata, Y. & Yamamoto, K. Angiotensin receptor blockers suppress antigen-specific T-cell responses and ameliorate collagen-induced arthritis in mice. Arthr. Rheumat. 52, 1920–1928 (2005).
Bahk, T. J., Daniels, M. D., Leon, J. S., Wang, K. & Engman, D. M. Comparison of angiotensin converting enzyme inhibition and angiotensin II receptor blockade for the prevention of experimental autoimmune myocarditis. Int. J. Cardiol. 125, 85–93 (2008).
Chang, Y. & Wei, W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin. Exp. Immunol. 179, 137–145 (2015).
Martin, M. F. et al. Captopril: a new treatment for rheumatoid arthritis? Lancet 1, 1325–1328 (1984).
Danilov, S. M. et al. Angiotensin-converting enzyme (CD143) is abundantly expressed by dendritic cells and discriminates human monocyte-derived dendritic cells from acute myeloid leukemia-derived dendritic cells. Exp. Hematol. 31, 1301–1309 (2003).
Viinikainen, A., Nyman, T., Fyhrquist, F. & Saijonmaa, O. Downregulation of angiotensin converting enzyme by TNF-alpha in differentiating human macrophages. Cytokine 18, 304–310 (2002).
Inagami, T. in Biochemical Regulation of Blood Pressure (ed. Soffer, R. L. ) 39–72 (John Wiley and Sons, New York, 1981).
Sun, X. et al. Catabolic attacks of membrane-bound angiotensin-converting enzyme on the N-terminal part of species-specific amyloid-beta peptides. Eur. J. Pharmacol. 588, 18–25 (2008).
Chen, H. L., Lünsdorf, H., Hecht, H. J. & Tsai, H. Porcine pulmonary angiotensin I-converting enzyme—biochemical characterization and spatial arrangement of the N- and C-domains by three-dimensional electron microscopic reconstruction. Micron 41, 674–685 (2010).
Allinson, T. M. et al. The role of ADAM10 and ADAM17 in the ectodomain shedding of angiotensin converting enzyme and the amyloid precursor protein. Eur. J. Biochem. 271, 2539–2547 (2004).
Parkin, E. T., Turner, A. J. & Hooper, N. M. Secretase-mediated cell surface shedding of the angiotensin-converting enzyme. Protein Pept. Lett. 11, 423–432 (2004).
Barauna, V. G., Campos, L. C., Miyakawa, A. A. & Krieger, J. E. ACE as a mechanosensor to shear stress influences the control of its own regulation via phosphorylation of cytoplasmic Ser(1270). PLoS ONE. 6, e22803 (2011).
Fleming, I. Signaling by the angiotensin-converting enzyme. Circ. Res. 98, 887–896 (2006).
Howard, T. E., Shai, S.-Y., Langford, K. G. & Martin, B. M. & Bernstein, K. E. Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol. Cell. Biol. 10, 4294–4302 (1990).
Wei, L., Alhenc-Gelas, F., Corvol, P. & Clauser, E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 266, 9002–9008 (1991).
Fuchs, S. et al. Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension 51, 267–274 (2008).
Rousseau, A. et al. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J. Biol. Chem. 270, 3656–3661 (1995).
Zhu, L. et al. Ac-SDKP suppresses TNF-α-induced ICAM-1 expression in endothelial cells via inhibition of IκB kinase and NF-κB activation. Am. J. Physiol. Heart Circ. Physiol. 310, H1176–H1183 (2016).
Hubert, C., Houot, A. M., Corvol, P. & Soubrier, F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J. Biol. Chem. 266, 15377–15383 (1991).
[No authors listed.] Alzheimer's Disease Facts and Figures. Alzheimer's Association https://www.alz.org/facts/ (2017)
Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 12, 459–509 (2016).
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 8, 595–608 (2016).
Schwartz, M. Can immunotherapy treat neurodegeneration? Science 357, 254–255 (2017).
Bernstein, K. E. et al. Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer's-like cognitive decline. J. Clin. Invest. 124, 1000–1012 (2014).
Acknowledgements
The authors thank B. Taylor of Cedars-Sinai Medical Center for help in preparing the manuscript before submission. The authors' work described in this Review was supported by US National Institute of Health grants P01HL129941, R21AI114965 and R03DK101592, and American Heart Association grants 16SDG30130015 and 17GRNT33661206.
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the discussion of the content. K.E.B., Z.K., D.-Y.C. and X.Z.S. researched data for the article. K.E.B., J.F.G., E.A.B. and X.Z.S. drafted and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (figure)
Angiotensinogen knockout mice were bred such that their ACE genes were either homozygous for the ACE 10 mutation (10/10) or WT for ACE. (PDF 66 kb)
Supplementary information S2 (figure)
A mouse model of Alzheimer's disease was crossed such that the ACE genes were either WT or ACE 10/10. (PDF 1919 kb)
Glossary
- ACE inhibitors
-
Compounds that block the formation of angiotensin II and all other angiotensin-converting enzyme (ACE) products by inhibiting ACE activity.
- Sarcoidosis
-
A granulomatous disease characterized by abnormal foci of inflammation. This disease is often associated with high plasma levels of angiotensin-converting enzyme.
- Type 1 angiotensin II receptors
-
(AT1Rs). Receptors for angiotensin II that mediate a variety of functions, such as decreased renal blood flow. Whereas humans have a single gene for this receptor (AGTR1), mice have two genes (Agtr1a and Agtr1b), which encode proteins that are referred to as AT1A and AT1B, respectively. Studies in Agtr1a−/− mice have demonstrated that most angiotensin II-mediated effects in the kidney are mediated by AT1A.
- ACE-like enzymes
-
Enzymes that have protein sequence similarity to human or mouse angiotensin-converting enzyme (ACE). Such enzymes usually bind zinc and have two catalytic domains.
- Granulomas
-
Compact aggregates of immune cells in response to infection or another form of chronic stimulation, resulting in a physical barrier surrounding the enclosed bacteria and hindering the spread of the pathogen throughout the organism. Tuberculosis is most commonly associated with granulomas, but a number of other infectious and noninfectious diseases induce granulomas, as do difficult-to-phagocytize inert foreign bodies such as those in berylliosis.
- Histoplasmosis
-
A granulomatous disease, often primary of the lung, caused by the fungus Histoplasma capsulatum.
- Leprosy
-
A granulomatous disease caused by Mycobacterium leprae; also known as Hansen disease.
- Silicosis
-
A granulomatous disease, often primary of the lung, caused by exposure to silica dust.
- Granulomatosis with polyangiitis
-
A systemic form of vasculitis (inflammation of blood vessels) often referred to as Wegener granulomatosis. The vasculitis typically involves small-to-medium-sized blood vessels that become inflamed and can develop granulomas. The origin of the disease is thought to be due to anti-neutrophil cytoplasmic antibodies (ANCAs).
- Schistosomiasis
-
A parasitic infection caused by the flatworm genus Schistosoma. Eggs of these worms can induce a granulomatous inflammatory response.
- ACE 10/10 mice
-
Mice homozygous for the angiotensin-converting enzyme (ACE) 10 mutation, resulting in ACE overexpression in myeloid cells, particularly in monocytes and macrophages. These animals develop normally and have normal blood pressure because ACE present on the surface of monocytes and macrophages, as well as circulating ACE shed from these cells, maintains normal angiotensin II plasma levels.
- B16 melanoma
-
An aggressive mouse tumour cell line that, when implanted into the skin, develops into a 600 mm3 lesion in ∼2 weeks.
- Syngeneic mice
-
Mice that can donate tissue or cells without triggering an immune response in the recipient mice because cellular proteins and the derived cell surface major histocompatibility complex (MHC) class I peptides are identical.
- Ovalbumin
-
(OVA). A main protein found in egg white. OVA is often used in immunology studies, as many reagents are commercially available, and many details are known as to how the protein stimulates an immune response.
- NeuACE mice
-
Mice that have been genetically modified to overexpress angiotensin-converting enzyme (ACE) in neutrophils. Unlike ACE 10/10 mice, NeuACE mice retain normal levels of ACE expression in all other tissues and cells, including lungs, kidneys, monocytes and macrophages. These animals also develop normally and have normal blood pressure.
- Anti-neutrophil antisera
-
Antibodies that recognize and attach to neutrophils, leading to neutrophil depletion.
- Amyloid plaques
-
Extracellular collections of amyloid protein in the brain. Such plaques are typical of Alzheimer disease.
- Alzheimer-prone mice
-
Mice that have been genetically modified to produce mutant versions of amyloid precursor protein and presenilin owing to expression of transgenic APPK595N,M596L and PS1ΔE9. These mice develop amyloid plaques in the brain over time and show a decreased ability to learn new tasks.
- Barnes maze test
-
A test involving a white platform with 20 equally spaced holes. Only one hole leads to an escape box, whereas the other holes lead to boxes that are too small to enter. The mice are trained to enter the escape box over several days. By measuring the time an animal takes to enter the escape box, and by measuring how rapidly knowledge of the location of the escape box is lost over several days, one can estimate learning and memory retention.
Rights and permissions
About this article
Cite this article
Bernstein, K., Khan, Z., Giani, J. et al. Angiotensin-converting enzyme in innate and adaptive immunity. Nat Rev Nephrol 14, 325–336 (2018). https://doi.org/10.1038/nrneph.2018.15
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2018.15
This article is cited by
-
Genetic proxies for antihypertensive drugs and mental disorders: Mendelian randomization study in European and East Asian populations
BMC Medicine (2024)
-
Comparative estimation of the effects of antihypertensive medications on schizophrenia occurrence: a multinational observational cohort study
BMC Psychiatry (2024)
-
Immunomodulation and immunopharmacology in heart failure
Nature Reviews Cardiology (2024)
-
Genetic Variations Related to Angiotensin II Production and Risk for Basal Cell Carcinoma
Biochemical Genetics (2024)
-
The neuroimmune axis of Alzheimer’s disease
Genome Medicine (2023)