Key Points
-
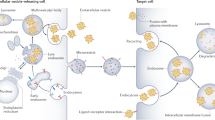
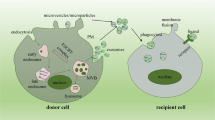
Extracellular vesicles are involved in cell–to–cell communication and they transfer nucleic acids, proteins and lipids that can alter the phenotype of the recipient cell
-
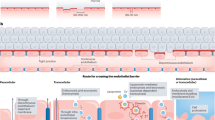
Extracellular vesicles can be used as biomarkers of renal diseases
-
Circulating microvesicles and exosomes may contribute to the development of renal diseases by immunomodulation, thrombogenesis and matrix modulation
-
Extracellular vesicles may have a therapeutic role in tissue regeneration after acute kidney injury
-
Extracellular vesicles have the potential to transfer endogenous and exogenous therapeutic substances to recipient cells
Abstract
Extracellular vesicles, such as exosomes and microvesicles, are host cell-derived packages of information that allow cell–cell communication and enable cells to rid themselves of unwanted substances. The release and uptake of extracellular vesicles has important physiological functions and may also contribute to the development and propagation of inflammatory, vascular, malignant, infectious and neurodegenerative diseases. This Review describes the different types of extracellular vesicles, how they are detected and the mechanisms by which they communicate with cells and transfer information. We also describe their physiological functions in cellular interactions, such as in thrombosis, immune modulation, cell proliferation, tissue regeneration and matrix modulation, with an emphasis on renal processes. We discuss how the detection of extracellular vesicles could be utilized as biomarkers of renal disease and how they might contribute to disease processes in the kidney, such as in acute kidney injury, chronic kidney disease, renal transplantation, thrombotic microangiopathies, vasculitides, IgA nephropathy, nephrotic syndrome, urinary tract infection, cystic kidney disease and tubulopathies. Finally, we consider how the release or uptake of extracellular vesicles can be blocked, as well as the associated benefits and risks, and how extracellular vesicles might be used to treat renal diseases by delivering therapeutics to specific cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
28 July 2017
This article has been updated to remove some characters that were accidently inserted at the end of a word. The error did not impact on the scientific meaning.
References
Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Morel, O., Jesel, L., Freyssinet, J. M. & Toti, F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 31, 15–26 (2011).
Ståhl, A. L., Sartz, L. & Karpman, D. Complement activation on platelet–leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood 117, 5503–5513 (2011). The paper presents complement-coated microvesicles in the circulation during HUS, suggesting a role in inflammation and thrombogenesis.
Iida, K., Whitlow, M. B. & Nussenzweig, V. Membrane vesiculation protects erythrocytes from destruction by complement. J. Immunol. 147, 2638–2642 (1991).
Mause, S. F. & Weber, C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 107, 1047–1057 (2010).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007). Seminal paper demonstrating the capability of exosomes to transfer genetic material, even between species.
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A. & Ratajczak, M. Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495 (2006).
Deregibus, M. C. et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110, 2440–2448 (2007).
Davizon, P., Munday, A. D. & Lopez, J. A. Tissue factor, lipid rafts, and microparticles. Semin. Thromb. Hemost. 36, 857–864 (2010).
Del Conde, I., Shrimpton, C. N., Thiagarajan, P. & Lopez, J. A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106, 1604–1611 (2005). Describes the importance of tissue factor and lipid rafts on microvesicles.
Sinauridze, E. I. et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 97, 425–434 (2007).
Pluskota, E. et al. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood 112, 2327–2335 (2008).
Meckes, D. G. Jr & Raab-Traub, N. Microvesicles and viral infection. J. Virol. 85, 12844–12854 (2011).
Ståhl, A. L. et al. A novel mechanism of bacterial toxin transfer within host blood cell-derived microvesicles. PLoS Pathog. 11, e1004619 (2015). This paper describes a novel mechanism of bacterial virulence whereby host-derived blood cell microvesicles transfer a bacterial virulence factor to the kidneys.
Keller, S. et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 72, 1095–1102 (2007).
Nilsson, J. et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br. J. Cancer 100, 1603–1607 (2009).
Stegmayr, B. & Ronquist, G. Promotive effect on human sperm progressive motility by prostasomes. Urol. Res. 10, 253–257 (1982).
Street, J. M. et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl Med. 10, 5 (2012).
Gyorgy, B. et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE 7, e49726 (2012).
Admyre, C. et al. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978 (2007).
Michael, A. et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 16, 34–38 (2010).
Severino, V. et al. Extracellular vesicles in bile as markers of malignant biliary stenoses. Gastroenterology http://dx.doi.org/10.1053/j.gastro.2017.04.043 (2017).
Andre, F. et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360, 295–305 (2002).
van Balkom, B. W., Pisitkun, T., Verhaar, M. C. & Knepper, M. A. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int. 80, 1138–1145 (2011). An excellent review of exosomes in renal diseases.
Skokos, D. et al. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 166, 868–876 (2001).
Combes, V. et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Invest. 104, 93–102 (1999).
Sapet, C. et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 108, 1868–1876 (2006).
Faure, V. et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J. Thromb. Haemost. 4, 566–573 (2006).
Cocucci, E., Racchetti, G. & Meldolesi, J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43–51 (2009).
Bianco, F. et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 174, 7268–7277 (2005).
Pizzirani, C. et al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 109, 3856–3864 (2007).
Tati, R. et al. Complement activation associated with ADAMTS13 deficiency in human and murine thrombotic microangiopathy. J. Immunol. 191, 2184–2193 (2013). This paper describes the release of complement-coated endothelial-derived microvesicles when plasma from patients with TTP is perfused over glomerular endothelial cells.
Park, J. E. et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteomics 9, 1085–1099 (2010).
Lehmann, B. D. et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 68, 7864–7871 (2008).
El Andaloussi, S., Mager, I., Breakefield, X. O. & Wood, M. J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12, 347–357 (2013). An excellent review covering the potential of extracellular vesicles in therapeutics.
Adell, M. A. et al. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J. Cell Biol. 205, 33–49 (2014).
Guescini, M., Genedani, S., Stocchi, V. & Agnati, L. F. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. (Vienna) 117, 1–4 (2010).
Colombo, M. et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 126, 5553–5565 (2013).
Crescitelli, R. et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2, 20677 (2013).
Boulanger, C. M., Amabile, N. & Tedgui, A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension 48, 180–186 (2006).
Chironi, G. N. et al. Endothelial microparticles in diseases. Cell Tissue Res. 335, 143–151 (2009).
Muralidharan-Chari, V. et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875–1885 (2009).
Daleke, D. L. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 44, 233–242 (2003).
Bevers, E. M. & Williamson, P. L. Phospholipid scramblase: an update. FEBS Lett. 584, 2724–2730 (2010).
Suzuki, J., Umeda, M., Sims, P. J. & Nagata, S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838 (2010).
Yang, H. et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151, 111–122 (2012).
Fujii, T., Sakata, A., Nishimura, S., Eto, K. & Nagata, S. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc. Natl Acad. Sci. USA 112, 12800–12805 (2015).
Kim, D. K. et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31, 933–939 (2015).
Lai, R. C. et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 5, 29828 (2016).
Jimenez, J. J. et al. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb. Res. 109, 175–180 (2003).
de Gassart, A., Geminard, C., Fevrier, B., Raposo, G. & Vidal, M. Lipid raft-associated protein sorting in exosomes. Blood 102, 4336–4344 (2003).
Biro, E. et al. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J. Thromb. Haemost. 3, 2754–2763 (2005).
Yanez-Mo, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Mulcahy, L. A., Pink, R. C. & Carter, D. R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, 24641 (2014).
George, J. N., Thoi, L. L., McManus, L. M. & Reimann, T. A. Isolation of human platelet membrane microparticles from plasma and serum. Blood 60, 834–840 (1982).
Arvidsson, I. et al. Shiga toxin-induced complement-mediated hemolysis and release of complement-coated red blood cell-derived microvesicles in hemolytic uremic syndrome. J. Immunol. 194, 2309–2318 (2015). The paper describes the involvement of red blood cell-derived microvesicles in haemolysis and inhibition by purinergic receptor inhibitors.
Mossberg, M. et al. C1-inhibitor decreases the release of vasculitis-like chemotactic endothelial microvesicles. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2016060637 (2017). This paper describes the chemotactic potential of endothelial-derived microvesicles positive for both kinin receptors, B1 and B2, and IL-8, and inhibition of their release by C1 inhibitor.
Fang, D. Y., King, H. W., Li, J. Y. & Gleadle, J. M. Exosomes and the kidney: blaming the messenger. Nephrology (Carlton) 18, 1–10 (2013).
Zhou, H. et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 74, 613–621 (2008).
Dear, J. W., Street, J. M. & Bailey, M. A. Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 13, 1572–1580 (2013).
Pisitkun, T., Shen, R. F. & Knepper, M. A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl Acad. Sci. USA 101, 13368–13373 (2004). A comprehensive study of exosomes in urine.
Erdbrugger, U. & Le, T. H. Extracellular vesicles in renal diseases: more than novel biomarkers? J. Am. Soc. Nephrol. 27, 12–26 (2016).
van der Pol, E. et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 12, 1182–1192 (2014).
Erdbrugger, U. & Lannigan, J. Analytical challenges of extracellular vesicle detection: a comparison of different techniques. Cytometry A 89, 123–134 (2016). A review describing and comparing methods for detection of extracellular vesicles.
Maas, S. L. et al. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J. Control. Release 200, 87–96 (2015).
Oosthuyzen, W. et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J. Physiol. 591, 5833–5842 (2013).
Murakami, T. et al. Development of glomerulus-, tubule-, and collecting duct-specific mRNA assay in human urinary exosomes and microvesicles. PLoS ONE 9, e109074 (2014).
Rood, I. M. et al. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 78, 810–816 (2010).
Salih, M., Zietse, R. & Hoorn, E. J. Urinary extracellular vesicles and the kidney: biomarkers and beyond. Am. J. Physiol. Renal Physiol. 306, F1251–F1259 (2014).
Wang, D. & Sun, W. Urinary extracellular microvesicles: isolation methods and prospects for urinary proteome. Proteomics 14, 1922–1932 (2014).
Tricarico, C., Clancy, J. & D'Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases http://dx.doi.org/10.1080/21541248.2016.1215283 (2016).
Clancy, J. W., Tricarico, C. J. & D'Souza-Schorey, C. Tumor-derived microvesicles in the tumor microenvironment: how vesicle heterogeneity can shape the future of a rapidly expanding field. Bioessays 37, 1309–1316 (2015).
Laulagnier, K. et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380, 161–171 (2004).
Bolukbasi, M. F. et al. miR-1289 and “zipcode”-like sequence enrich mRNAs in microvesicles. Mol. Ther. Nucleic Acids 1, e10 (2012).
Alexy, T., Rooney, K., Weber, M., Gray, W. D. & Searles, C. D. TNF-alpha alters the release and transfer of microparticle-encapsulated miRNAs from endothelial cells. Physiol. Genomics 46, 833–840 (2014).
Parolini, I. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 (2009).
Nolte-'t Hoen, E. N., Buschow, S. I., Anderton, S. M., Stoorvogel, W. & Wauben, M. H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 113, 1977–1981 (2009).
Dasgupta, S. K., Le, A., Chavakis, T., Rumbaut, R. E. & Thiagarajan, P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation 125, 1664–1672 (2012).
Collino, F. et al. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J. Am. Soc. Nephrol. 26, 2349–2360 (2015).
Quesenberry, P. J. et al. Cellular phenotype and extracellular vesicles: basic and clinical considerations. Stem Cells Dev. 23, 1429–1436 (2014).
Pegtel, D. M. et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107, 6328–6333 (2010).
Balaj, L. et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 (2011).
Thakur, B. K. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24, 766–769 (2014).
Kahlert, C. et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 289, 3869–3875 (2014).
Miranda, K. C. et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 78, 191–199 (2010).
Muhsin-Sharafaldine, M. R. et al. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget 7, 56279–56294 (2016).
Spees, J. L., Olson, S. D., Whitney, M. J. & Prockop, D. J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288 (2006).
Phinney, D. G. et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 6, 8472 (2015).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016).
Mack, M. et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 6, 769–775 (2000). The first description of microvesicles transferring functional receptors involved in inflammatory signalling.
Al-Nedawi, K., Meehan, B., Kerbel, R. S., Allison, A. C. & Rak, J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl Acad. Sci. USA 106, 3794–3799 (2009).
Rozmyslowicz, T. et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 17, 33–42 (2003). This paper describes microvesicle transfer of functional receptors involved in inflammatory signalling.
Baj-Krzyworzeka, M. et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp. Hematol. 30, 450–459 (2002).
Salanova, B. et al. Beta2-integrins and acquired glycoprotein IIb/IIIa (GPIIb/IIIa) receptors cooperate in NF-kappaB activation of human neutrophils. J. Biol. Chem. 282, 27960–27969 (2007).
Kahn, R. et al. Microvesicle transfer of kinin B1-receptors is a novel inflammatory mechanism in vasculitis. Kidney Int. 91, 96–105 (2017). This paper describes transfer of functional kinin receptors between cells by microvesicles.
Janowska-Wieczorek, A. et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood 98, 3143–3149 (2001).
Barry, O. P., Pratico, D., Lawson, J. A. & FitzGerald, G. A. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Invest. 99, 2118–2127 (1997).
Giri, P. K. & Schorey, J. S. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS ONE 3, e2461 (2008).
Walker, J. D., Maier, C. L. & Pober, J. S. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. J. Immunol. 182, 1548–1559 (2009).
Gould, S. J., Booth, A. M. & Hildreth, J. E. The Trojan exosome hypothesis. Proc. Natl Acad. Sci. USA 100, 10592–10597 (2003).
Chaput, N. & Thery, C. Exosomes: immune properties and potential clinical implementations. Semin. Immunopathol. 33, 419–440 (2011).
Mesri, M. & Altieri, D. C. Endothelial cell activation by leukocyte microparticles. J. Immunol. 161, 4382–4387 (1998).
Gasser, O. & Schifferli, J. A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104, 2543–2548 (2004).
Distler, J. H., Huber, L. C., Gay, S., Distler, O. & Pisetsky, D. S. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity 39, 683–690 (2006).
Burrello, J. et al. Stem cell-derived extracellular vesicles and immune-modulation. Front. Cell Dev. Biol. 4, 83 (2016).
Peche, H., Heslan, M., Usal, C., Amigorena, S. & Cuturi, M. C. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation 76, 1503–1510 (2003).
Miksa, M. et al. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock 25, 586–593 (2006).
Sadallah, S., Eken, C., Martin, P. J. & Schifferli, J. A. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J. Immunol. 186, 6543–6552 (2011).
Sprague, D. L. et al. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood 111, 5028–5036 (2008).
Brown, G. T. & McIntyre, T. M. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1beta-rich microparticles. J. Immunol. 186, 5489–5496 (2011).
Barry, O. P., Pratico, D., Savani, R. C. & FitzGerald, G. A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Invest. 102, 136–144 (1998).
Mause, S. F., von Hundelshausen, P., Zernecke, A., Koenen, R. R. & Weber, C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 25, 1512–1518 (2005).
Sims, P. J., Faioni, E. M., Wiedmer, T. & Shattil, S. J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 263, 18205–18212 (1988).
Ståhl, A. L. et al. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood 111, 5307–5315 (2008). This paper describes complement-coated platelets releasing tissue factor-positive microvesicles and inhibition by addition of factor H to prevent complement activation on blood cells in atypical HUS.
Yin, W., Ghebrehiwet, B. & Peerschke, E. I. Expression of complement components and inhibitors on platelet microparticles. Platelets 19, 225–233 (2008).
Rabesandratana, H., Toutant, J. P., Reggio, H. & Vidal, M. Decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59) are released within exosomes during in vitro maturation of reticulocytes. Blood 91, 2573–2580 (1998).
Bevers, E. M. & Williamson, P. L. Getting to the outer leaflet: physiology of phosphatidylserine exposure at the plasma membrane. Physiol. Rev. 96, 605–645 (2016).
Falati, S. et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 197, 1585–1598 (2003).
Ståhl, A. L., Sartz, L., Nelsson, A., Békássy, Z. D. & Karpman, D. Shiga toxin and lipopolysaccharide induce platelet-leukocyte aggregates and tissue factor release, a thrombotic mechanism in hemolytic uremic syndrome. PLoS ONE 4, e6990 (2009). The presence of tissue factor on blood cell-derived microvesicles could contribute to thrombotic microangiopathy.
Abid Hussein, M. N. et al. Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb. Res. 121, 865–871 (2008).
Sabatier, F. et al. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 99, 3962–3970 (2002).
Raturi, A., Miersch, S., Hudson, J. W. & Mutus, B. Platelet microparticle-associated protein disulfide isomerase promotes platelet aggregation and inactivates insulin. Biochim. Biophys. Acta 1778, 2790–2796 (2008).
Gilbert, G. E. et al. Platelet-derived microparticles express high affinity receptors for factor VIII. J. Biol. Chem. 266, 17261–17268 (1991).
Van Der Meijden, P. E. et al. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 10, 1355–1362 (2012).
Connor, D. E., Exner, T., Ma, D. D. & Joseph, J. E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 103, 1044–1052 (2010).
Rossaint, J. et al. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 7, 13464 (2016).
Berckmans, R. J. et al. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 85, 639–646 (2001).
Tans, G. et al. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood 77, 2641–2648 (1991).
Abid Hussein, M. N., Boing, A. N., Sturk, A., Hau, C. M. & Nieuwland, R. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb. Haemost. 98, 1096–1107 (2007).
Perez-Casal, M., Downey, C., Fukudome, K., Marx, G. & Toh, C. H. Activated protein C induces the release of microparticle-associated endothelial protein C receptor. Blood 105, 1515–1522 (2005).
Perez-Casal, M. et al. Microparticle-associated endothelial protein C receptor and the induction of cytoprotective and anti-inflammatory effects. Haematologica 94, 387–394 (2009).
Janowska-Wieczorek, A. et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer 113, 752–760 (2005).
Hood, J. L. et al. Paracrine induction of endothelium by tumor exosomes. Lab. Invest. 89, 1317–1328 (2009).
Mezentsev, A. et al. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 289, H1106–H1114 (2005).
Kim, H. K., Song, K. S., Chung, J. H., Lee, K. R. & Lee, S. N. Platelet microparticles induce angiogenesis in vitro. Br. J. Haematol. 124, 376–384 (2004).
Chen, J. et al. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS ONE 9, e115316 (2014).
Brill, A., Dashevsky, O., Rivo, J., Gozal, Y. & Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 67, 30–38 (2005).
Riazifar, M., Pone, E. J., Lotvall, J. & Zhao, W. Stem cell extracellular vesicles: extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 57, 125–154 (2017).
Aliotta, J. M. et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells 25, 2245–2256 (2007).
Sedgwick, A. E., Clancy, J. W., Olivia Balmert, M. & D'Souza-Schorey, C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci. Rep. 5, 14748 (2015).
Graves, L. E. et al. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 64, 7045–7049 (2004).
Clancy, J. W. et al. Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat. Commun. 6, 6919 (2015).
McCready, J., Sims, J. D., Chan, D. & Jay, D. G. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 10, 294 (2010).
Bruno, S. et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067 (2009).
Oosthuyzen, W. et al. Vasopressin regulates extracellular vesicle uptake by kidney collecting duct cells. J. Am. Soc. Nephrol. 27, 3345–3355 (2016).
Cheng, Y. et al. A translational study of urine miRNAs in acute myocardial infarction. J. Mol. Cell. Cardiol. 53, 668–676 (2012).
Gonzales, P. A. et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 20, 363–379 (2009).
Street, J. M. et al. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 589, 6119–6127 (2011).
Winyard, P. J. & Price, K. L. Experimental renal progenitor cells: repairing and recreating kidneys? Pediatr. Nephrol. 29, 665–672 (2014).
Ranghino, A. et al. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res. Ther. 8, 24 (2017).
Turco, A. E. et al. Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J. Extracell. Vesicles 5, 29642 (2016).
Karpman, D., Loos, S., Tati, R. & Arvidsson, I. Haemolytic uraemic syndrome. J. Intern. Med. 281, 123–148 (2017).
Ge, S. et al. Microparticle generation and leucocyte death in Shiga toxin-mediated HUS. Nephrol. Dial. Transplant. 27, 2768–2775 (2012).
Brigotti, M. et al. Clinical relevance of shiga toxin concentrations in the blood of patients with hemolytic uremic syndrome. Pediatr. Infect. Dis. J. 30, 486–490 (2011).
Karpman, D. et al. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood 97, 3100–3108 (2001).
Zoja, C., Buelli, S. & Morigi, M. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr. Nephrol. 25, 2231–2240 (2010).
Afshar-Kharghan, V. Unleashed platelets in aHUS. Blood 111, 5266 (2008).
Levy, G. G. et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 413, 488–494 (2001).
Manea, M. & Karpman, D. Molecular basis of ADAMTS13 dysfunction in thrombotic thrombocytopenic purpura. Pediatr. Nephrol. 24, 447–458 (2009).
Tsai, H. M. Pathophysiology of thrombotic thrombocytopenic purpura. Int. J. Hematol. 91, 1–19 (2010).
Kelton, J. G., Warkentin, T. E., Hayward, C. P., Murphy, W. G. & Moore, J. C. Calpain activity in patients with thrombotic thrombocytopenic purpura is associated with platelet microparticles. Blood 80, 2246–2251 (1992).
Jimenez, J. J. et al. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br. J. Haematol. 123, 896–902 (2003).
Brogan, P. A. et al. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum. 50, 927–936 (2004).
Erdbruegger, U. et al. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology (Oxford) 47, 1820–1825 (2008).
Clarke, L. A. et al. Endothelial injury and repair in systemic vasculitis of the young. Arthritis Rheum. 62, 1770–1780 (2010).
Daniel, L. et al. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 69, 1416–1423 (2006).
Hong, Y. et al. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J. Am. Soc. Nephrol. 23, 49–62 (2012).
Gasser, O. et al. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 285, 243–257 (2003).
Hogan, M. C. et al. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int. 85, 1225–1237 (2014).
Huang, Y. M., Wang, H., Wang, C., Chen, M. & Zhao, M. H. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 67, 2780–2790 (2015).
Eleftheriou, D., Hong, Y., Klein, N. J. & Brogan, P. A. Thromboembolic disease in systemic vasculitis is associated with enhanced microparticle-mediated thrombin generation. J. Thromb. Haemost. 9, 1864–1867 (2011).
Kahn, R. et al. Contact-system activation in children with vasculitis. Lancet 360, 535–541 (2002).
Kahn, R. et al. Neutrophil-derived proteinase 3 induces kallikrein-independent release of a novel vasoactive kinin. J. Immunol. 182, 7906–7915 (2009).
Mack, M. Leukocyte-derived microvesicles dock on glomerular endothelial cells: stardust in the kidney. Kidney Int. 91, 13–15 (2017).
Duan, Z. Y. et al. Selection of urinary sediment miRNAs as specific biomarkers of IgA nephropathy. Sci. Rep. 6, 23498 (2016). A paper describing urinary erythrocyte miRNAs derived from microvesicles as biomarkers of IgA nephropathy.
Wang, G. et al. Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis. Markers 30, 171–179 (2011).
Moon, P. G. et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 11, 2459–2475 (2011).
Zhou, H. et al. Urinary exosomal Wilms' tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Renal Physiol. 305, F553–F559 (2013).
Lee, H. et al. Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr. Nephrol. 27, 317–320 (2012).
Rood, I. M. et al. Increased expression of lysosome membrane protein 2 in glomeruli of patients with idiopathic membranous nephropathy. Proteomics 15, 3722–3730 (2015).
Gao, C. et al. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb. Haemost. 107, 681–689 (2012).
Eyre, J. et al. Monocyte- and endothelial-derived microparticles induce an inflammatory phenotype in human podocytes. Nephron Exp. Nephrol. 119, e58–e66 (2011).
Woei, A. J. F. J. et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb. Res. 133, 799–803 (2014).
Hiemstra, T. F. et al. Human urinary exosomes as innate immune effectors. J. Am. Soc. Nephrol. 25, 2017–2027 (2014).
Hogan, M. C. et al. Identification of biomarkers for PKD1 using urinary exosomes. J. Am. Soc. Nephrol. 26, 1661–1670 (2015).
Hogan, M. C. et al. Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 20, 278–288 (2009).
Ben-Dov, I. Z. et al. Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: description of miRNA profiles at baseline. PLoS ONE 9, e86856 (2014).
Joo, K. W. et al. Reduced urinary excretion of thiazide-sensitive Na-Cl cotransporter in Gitelman syndrome: preliminary data. Am. J. Kidney Dis. 50, 765–773 (2007).
Tokes-Fuzesi, M. et al. Microparticles and acute renal dysfunction in septic patients. J. Crit. Care 28, 141–147 (2013).
Martino, F. et al. Circulating microRNAs are not eliminated by hemodialysis. PLoS ONE 7, e38269 (2012).
Cantaluppi, V. et al. Protective effect of resin adsorption on septic plasma-induced tubular injury. Crit. Care 14, R4 (2010).
Mariano, F. et al. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Crit. Care 12, R42 (2008).
du Cheyron, D. et al. Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am. J. Kidney Dis. 42, 497–506 (2003).
Zhou, H. et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 70, 1847–1857 (2006).
Chen, H. H. et al. Exosomal ATF3 RNA attenuates pro-inflammatory gene MCP-1 transcription in renal ischemia-reperfusion. J. Cell. Physiol. 229, 1202–1211 (2014).
Mostefai, H. A. et al. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am. J. Respir. Crit. Care Med. 178, 1148–1155 (2008).
Delabranche, X. et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 39, 1695–1703 (2013).
Soriano, A. O. et al. Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Crit. Care Med. 33, 2540–2546 (2005).
Trepesch, C. et al. High intravascular tissue factor-but not extracellular microvesicles-in septic patients is associated with a high SAPS II score. J. Intensive Care 4, 34 (2016).
Joop, K. et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb. Haemost. 85, 810–820 (2001).
Nieuwland, R. et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 95, 930–935 (2000).
Timar, C. I. et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 121, 510–518 (2013).
Oehmcke, S. et al. A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes. PLoS Pathog. 9, e1003529 (2013).
Mortaza, S. et al. Detrimental hemodynamic and inflammatory effects of microparticles originating from septic rats. Crit. Care Med. 37, 2045–2050 (2009).
Meziani, F., Delabranche, X., Asfar, P. & Toti, F. Bench-to-bedside review: circulating microparticles — a new player in sepsis? Crit. Care 14, 236 (2010).
Camussi, G., Cantaluppi, V., Deregibus, M. C., Gatti, E. & Tetta, C. Role of microvesicles in acute kidney injury. Contrib. Nephrol. 174, 191–199 (2011).
Bruno, S. & Camussi, G. Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Methods Mol. Biol. 879, 367–380 (2012).
Akyurekli, C. et al. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 11, 150–160 (2015).
He, J. et al. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 17, 493–500 (2012).
Biancone, L., Bruno, S., Deregibus, M. C., Tetta, C. & Camussi, G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant. 27, 3037–3042 (2012).
Bianchi, F., Sala, E., Donadei, C., Capelli, I. & La Manna, G. Potential advantages of acute kidney injury management by mesenchymal stem cells. World J. Stem Cells 6, 644–650 (2014).
Camussi, G., Deregibus, M. C., Bruno, S., Cantaluppi, V. & Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 78, 838–848 (2010). An excellent review about how microvesicles transfer information between cells.
Eirin, A. et al. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 6, 36120 (2016).
Ju, G. Q. et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE 10, e0121534 (2015).
Tomasoni, S. et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 22, 772–780 (2013).
Wang, Y., Lu, X., He, J. & Zhao, W. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res. Ther. 6, 100 (2015).
Hao, S., Yuan, J. & Xiang, J. Nonspecific CD4+ T cells with uptake of antigen-specific dendritic cell-released exosomes stimulate antigen-specific CD8+ CTL responses and long-term T cell memory. J. Leukoc. Biol. 82, 829–838 (2007).
Montecalvo, A. et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J. Immunol. 180, 3081–3090 (2008).
Grange, C. et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 33, 1055–1063 (2014).
Gatti, S. et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 26, 1474–1483 (2011).
Bonventre, J. V. Microvesicles from mesenchymal stromal cells protect against acute kidney injury. J. Am. Soc. Nephrol. 20, 927–928 (2009).
Cantaluppi, V. et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 82, 412–427 (2012).
Herrera Sanchez, M. B. et al. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res. Ther. 5, 124 (2014).
Ando, M. et al. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney Int. 62, 1757–1763 (2002).
Burton, J. O. et al. Elevated levels of procoagulant plasma microvesicles in dialysis patients. PLoS ONE 8, e72663 (2013).
Amabile, N. et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 16, 3381–3388 (2005).
Trappenburg, M. C. et al. Chronic renal failure is accompanied by endothelial activation and a large increase in microparticle numbers with reduced procoagulant capacity. Nephrol. Dial. Transplant. 27, 1446–1453 (2012).
Amabile, N., Guerin, A. P., Tedgui, A., Boulanger, C. M. & London, G. M. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrol. Dial. Transplant. 27, 1873–1880 (2012).
Boulanger, C. M. et al. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension 49, 902–908 (2007).
Lv, L. L. et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Renal Physiol. 305, F1220–F1227 (2013).
Lv, L. L. et al. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin. Chim. Acta 428, 26–31 (2014).
Al-Massarani, G. et al. Kidney transplantation decreases the level and procoagulant activity of circulating microparticles. Am. J. Transplant. 9, 550–557 (2009).
Dimuccio, V. et al. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS ONE 9, e104490 (2014).
Meehan, S. M. et al. Platelets and capillary injury in acute humoral rejection of renal allografts. Hum. Pathol. 34, 533–540 (2003).
Cumpelik, A. et al. Mechanism of platelet activation and hypercoagulability by antithymocyte globulins (ATG). Am. J. Transplant. 15, 2588–2601 (2015).
Renner, B. et al. Cyclosporine induces endothelial cell release of complement-activating microparticles. J. Am. Soc. Nephrol. 24, 1849–1862 (2013).
Matignon, M. et al. Urinary cell mRNA profiles and differential diagnosis of acute kidney graft dysfunction. J. Am. Soc. Nephrol. 25, 1586–1597 (2014).
Lorenzen, J. M. et al. Long noncoding RNAs in urine are detectable and may enable early detection of acute T cell-mediated rejection of renal allografts. Clin. Chem. 61, 1505–1514 (2015).
Pisitkun, T., Gandolfo, M. T., Das, S., Knepper, M. A. & Bagnasco, S. M. Application of systems biology principles to protein biomarker discovery: urinary exosomal proteome in renal transplantation. Proteomics Clin. Appl. 6, 268–278 (2012).
Alvarez, S. et al. Urinary exosomes as a source of kidney dysfunction biomarker in renal transplantation. Transplant. Proc. 45, 3719–3723 (2013).
Peake, P. W. et al. A comparison of the ability of levels of urinary biomarker proteins and exosomal mRNA to predict outcomes after renal transplantation. PLoS ONE 9, e98644 (2014).
Sonoda, H. et al. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 297, F1006–F1016 (2009).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
Janas, A. M., Sapon, K., Janas, T., Stowell, M. H. & Janas, T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim.Biophys. Acta 1858, 1139–1151 (2016).
Khalyfa, A. et al. Extracellular microvesicle microRNAs in children with sickle cell anaemia with divergent clinical phenotypes. Br. J. Haematol. 174, 786–798 (2016).
Gilani, S. I., Weissgerber, T. L., Garovic, V. D. & Jayachandran, M. Preeclampsia and extracellular vesicles. Curr. Hypertens. Rep. 18, 68 (2016).
Sellam, J. et al. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 11, R156 (2009).
Aatonen, M., Gronholm, M. & Siljander, P. R. Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin. Thromb. Hemost. 38, 102–113 (2012).
Nielsen, C. T., Ostergaard, O., Johnsen, C., Jacobsen, S. & Heegaard, N. H. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 63, 3067–3077 (2011).
Nielsen, C. T. et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 64, 1227–1236 (2012).
Nielsen, C. T., Rasmussen, N. S., Heegaard, N. H. & Jacobsen, S. “Kill” the messenger: targeting of cell-derived microparticles in lupus nephritis. Autoimmun. Rev. 15, 719–725 (2016).
Sole, C., Cortes-Hernandez, J., Felip, M. L., Vidal, M. & Ordi-Ros, J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol. Dial. Transplant. 30, 1488–1496 (2015).
Knijff-Dutmer, E. A., Koerts, J., Nieuwland, R., Kalsbeek-Batenburg, E. M. & van de Laar, M. A. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 46, 1498–1503 (2002).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Rectenwald, J. E. et al. D-Dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis. A pilot study. Thromb. Haemost. 94, 1312–1317 (2005).
Chirinos, J. A. et al. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J. Am. Coll. Cardiol. 45, 1467–1471 (2005).
Campello, E., Spiezia, L., Radu, C. M. & Simioni, P. Microparticles as biomarkers of venous thromboembolic events. Biomark. Med. 10, 743–755 (2016).
Bal, L. et al. Factors influencing the level of circulating procoagulant microparticles in acute pulmonary embolism. Arch. Cardiovasc. Dis. 103, 394–403 (2010).
Dignat-George, F. et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb. Haemost. 91, 667–673 (2004).
Pericleous, C., Giles, I. & Rahman, A. Are endothelial microparticles potential markers of vascular dysfunction in the antiphospholipid syndrome? Lupus 18, 671–675 (2009).
Brodsky, R. A. Paroxysmal nocturnal hemoglobinuria. Blood 124, 2804–2811 (2014).
Helley, D. et al. Evaluation of hemostasis and endothelial function in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Haematologica 95, 574–581 (2010).
Angelillo-Scherrer, A. Leukocyte-derived microparticles in vascular homeostasis. Circ. Res. 110, 356–369 (2012).
Leroyer, A. S., Tedgui, A. & Boulanger, C. M. Role of microparticles in atherothrombosis. J. Intern. Med. 263, 528–537 (2008).
Rautou, P. E. et al. Microparticles, vascular function, and atherothrombosis. Circ. Res. 109, 593–606 (2011).
Owens, A. P. III & Mackman, N. Microparticles in hemostasis and thrombosis. Circ. Res. 108, 1284–1297 (2011).
Lakhter, A. J. & Sims, E. K. Minireview: emerging roles for extracellular vesicles in diabetes and related metabolic disorders. Mol. Endocrinol. 29, 1535–1548 (2015).
Omoto, S. et al. Detection of monocyte-derived microparticles in patients with type II diabetes mellitus. Diabetologia 45, 550–555 (2002).
Nomura, S. Dynamic role of microparticles in type 2 diabetes mellitus. Curr. Diabetes Rev. 5, 245–251 (2009).
Sabatier, F. et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 51, 2840–2845 (2002).
Diamant, M. et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 106, 2442–2447 (2002).
Chen, Y., Feng, B., Li, X., Ni, Y. & Luo, Y. Plasma endothelial microparticles and their correlation with the presence of hypertension and arterial stiffness in patients with type 2 diabetes. J. Clin. Hypertens. (Greenwich) 14, 455–460 (2012).
Zubiri, I. et al. Kidney tissue proteomics reveals regucalcin downregulation in response to diabetic nephropathy with reflection in urinary exosomes. Transl Res. 166, 474–484.e4 (2015).
Johansson, H. et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 54, 1755–1762 (2005).
Ueba, T. et al. Level, distribution and correlates of platelet-derived microparticles in healthy individuals with special reference to the metabolic syndrome. Thromb. Haemost. 100, 280–285 (2008).
Murakami, T. et al. Impact of weight reduction on production of platelet-derived microparticles and fibrinolytic parameters in obesity. Thromb. Res. 119, 45–53 (2007).
Heinrich, L. F., Andersen, D. K., Cleasby, M. E. & Lawson, C. Long-term high fat feeding of rats results in increased numbers of circulating microvesicles with pro-inflammatory effects on endothelial cells. Br. J. Nutr. 113, 1704–1711 (2015).
Stepanian, A. et al. Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring) 21, 2236–2243 (2013).
Agouni, A. et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am. J. Pathol. 173, 1210–1219 (2008).
Arteaga, R. B. et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am. J. Cardiol. 98, 70–74 (2006).
Little, K. M., Smalley, D. M., Harthun, N. L. & Ley, K. The plasma microparticle proteome. Semin. Thromb. Hemost. 36, 845–856 (2010).
Karolina, D. S. et al. Circulating miRNA profiles in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 97, E2271–E2276 (2012).
Preston, R. A. et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 41, 211–217 (2003).
Wang, J. M. et al. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J. Hum. Hypertens. 23, 307–315 (2009).
Huang, P. H. et al. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J. Hypertens. 28, 1655–1665 (2010).
Kwon, S. H. et al. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol. Dial. Transplant. 32, 800–807 (2016).
Wahlgren, J. et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 40, e130 (2012).
Ohno, S. I. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21, 185–191 (2013).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Tian, Y. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35, 2383–2390 (2014).
Johnsen, K. B. et al. A comprehensive overview of exosomes as drug delivery vehicles — endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta 1846, 75–87 (2014).
Pitt, J. M. et al. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Invest. 126, 1224–1232 (2016).
Lener, T. et al. Applying extracellular vesicles based therapeutics in clinical trials — an ISEV position paper. J. Extracell. Vesicles 4, 30087 (2015).
Rand, M. L., Wang, H., Bang, K. W., Packham, M. A. & Freedman, J. Rapid clearance of procoagulant platelet-derived microparticles from the circulation of rabbits. J. Thromb. Haemost. 4, 1621–1623 (2006).
Morel, O. et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler. Thromb. Vasc. Biol. 26, 2594–2604 (2006).
Nomura, S., Kanazawa, S. & Fukuhara, S. Effects of efonidipine on platelet and monocyte activation markers in hypertensive patients with and without type 2 diabetes mellitus. J. Hum. Hypertens. 16, 539–547 (2002).
Nomura, S. et al. Probucol and ticlopidine: effect on platelet and monocyte activation markers in hyperlipidemic patients with and without type 2 diabetes. Atherosclerosis 174, 329–335 (2004).
Nomura, S. et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets 20, 16–22 (2009).
Nomura, S. et al. Effects of eicosapentaenoic acid on endothelial cell-derived microparticles, angiopoietins and adiponectin in patients with type 2 diabetes. J. Atheroscler. Thromb. 16, 83–90 (2009).
Esposito, K., Ciotola, M. & Giugliano, D. Pioglitazone reduces endothelial microparticles in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 26, 1926 (2006).
Yano, Y. et al. The effects of calpeptin (a calpain specific inhibitor) on agonist induced microparticle formation from the platelet plasma membrane. Thromb. Res. 71, 385–396 (1993).
Zafrani, L. et al. Calpastatin controls polymicrobial sepsis by limiting procoagulant microparticle release. Am. J. Respir. Crit. Care Med. 185, 744–755 (2012).
Iero, M. et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 15, 80–88 (2008).
Chalmin, F. et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 120, 457–471 (2010).
Faille, D. et al. Endocytosis and intracellular processing of platelet microparticles by brain endothelial cells. J. Cell. Mol. Med. 16, 1731–1738 (2012).
Barres, C. et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 115, 696–705 (2010).
Acknowledgements
The authors gratefully acknowledge R. Tati (Department of Pediatrics, Lund University, Sweden) for help with figures 1 and 2. They are also grateful for funding from The Swedish Research Council (K2013-64X-14008-13-5 and K2015- 99X-22877-01-6), The Knut and Alice Wallenberg Foundation (Wallenberg Clinical Scholar 2015.0320), The Torsten Söderberg Foundation, Skåne Centre of Excellence in Health, Crown Princess Lovisa's Society for Child Care, Region Skåne and The Konung Gustaf V:s 80-årsfond.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussion of the content, and revising or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Karpman, D., Ståhl, Al. & Arvidsson, I. Extracellular vesicles in renal disease. Nat Rev Nephrol 13, 545–562 (2017). https://doi.org/10.1038/nrneph.2017.98
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.98
This article is cited by
-
The efficacy of extracellular vesicles for acute lung injury in preclinical animal models: a meta-analysis
BMC Pulmonary Medicine (2024)
-
Interorgan communication networks in the kidney–lung axis
Nature Reviews Nephrology (2024)
-
Kidney tubular epithelial cells control interstitial fibroblast fate by releasing TNFAIP8-encapsulated exosomes
Cell Death & Disease (2023)
-
Quercetin alleviates tubulointerstitial inflammation by inhibiting exosomes-mediated crosstalk between tubular epithelial cells and macrophages
Inflammation Research (2023)
-
Research progress of nephrotic syndrome accompanied by thromboembolism
International Urology and Nephrology (2023)