Abstract
Consensus definitions have been reached for both acute kidney injury (AKI) and chronic kidney disease (CKD) and these definitions are now routinely used in research and clinical practice. The KDIGO guideline defines AKI as an abrupt decrease in kidney function occurring over 7 days or less, whereas CKD is defined by the persistence of kidney disease for a period of >90 days. AKI and CKD are increasingly recognized as related entities and in some instances probably represent a continuum of the disease process. For patients in whom pathophysiologic processes are ongoing, the term acute kidney disease (AKD) has been proposed to define the course of disease after AKI; however, definitions of AKD and strategies for the management of patients with AKD are not currently available. In this consensus statement, the Acute Disease Quality Initiative (ADQI) proposes definitions, staging criteria for AKD, and strategies for the management of affected patients. We also make recommendations for areas of future research, which aim to improve understanding of the underlying processes and improve outcomes for patients with AKD.
Similar content being viewed by others
Main
Acute kidney injury (AKI) is estimated to occur in about 20–200 per million population in the community, 7–18% of patients in hospital, and approximately 50% of patients admitted to the intensive care unit (ICU)1,2. Importantly, AKI is associated with morbidity and mortality; an estimated 2 million people worldwide die of AKI every year, whereas AKI survivors are at increased risk of developing chronic kidney disease (CKD) and end-stage renal disease (ESRD) — conditions that carry a high economic, societal and personal burden3,4.
Over the past 15 years, consensus definitions have been reached for both AKI and CKD; these definitions are applied routinely for the diagnosis of these diseases in research and clinical practice5,6. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines define AKI as an abrupt decrease in kidney function that occurs over a period of 7 days or less, and CKD as abnormalities in kidney structure or function that persist for >90 days6. However, it is increasingly recognized that AKI and CKD are not always discrete entities and likely represent a continuum with patients who have sustained an episode of AKI having an increased risk of developing either de novo CKD or worsening of underlying CKD4 (Figs 1,2). In addition, the risk factors for AKI and CKD, such as advanced age, diabetes and hypertension, often overlap. The term acute kidney disease (AKD)5 has been proposed to define the course of disease after AKI among patients in whom the renal pathophysiologic processes are ongoing. Although AKI and CKD are well-characterized entities, AKD has not been systematically studied. As a prerequisite to the further study of AKD, the research community requires a common lexicon to standardize approaches and enable comparisons of different studies. Developing a common lexicon requires the formulation of a consensus definition of AKD, a staging system, and recommendations for approaches to clinical management. As the link between AKI and CKD is firmly established, the AKD period represents the time window wherein critical interventions might be initiated to alter the natural history of kidney disease. To develop a common framework and support further research for acute and progressive kidney disease, the 16th Acute Disease Quality Initiative (ADQI) sought to propose definitions and staging criteria for AKD and renal recovery and make recommendations for clinical practice and future research.
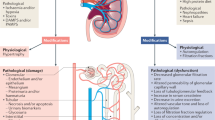
Acute kidney injury and chronic kidney disease often form a continuum of disease as opposed to being separate entities. The various disease modifiers and risk factors might represent opportunities to intervene and mitigate the poor outcomes associated with these diseases. Modified from Acute Dialysis Quality Initiative 16; www.adqi.org.
AKI, AKD and CKD can form a continuum whereby initial kidney injury can lead to persistent renal injury, eventually leading to CKD. AKI is defined as an abrupt decrease in kidney function occurring over 7 days or less, whereas CKD is defined by the persistence of kidney disease for a period of >90 days. AKD describes acute or subacute damage and/or loss of kidney function for a duration of between 7 and 90 days after exposure to an AKI initiating event. Recovery from AKI within 48 h of the initiating event typically heralds rapid reversal of AKI. For patients with pre-existing CKD, the AKI event can be superimposed on CKD, with AKD existing on a background of CKD. Patients who suffer AKD with pre-existing CKD are probably at high-risk of kidney disease progression. Modified from Acute Dialysis Quality Initiative 16; www.adqi.org.
Methods
The Conference Chairs of the 16th ADQI consensus committee (L.S.C., J.A.K. and C.R.) convened a diverse panel of clinicians and researchers representing relevant disciplines — internal medicine, primary care, nephrology, critical care, paediatrics, pharmacy, epidemiology, health-services research, biostatistics, bioinformatics and data analytics — from Europe, North America and Australia, to discuss the issues relating to persistent AKI and renal recovery. The conference was held over 2.5 days in San Diego, USA on November 8–10, 2015.
This consensus meeting followed the established ADQI process, and used a modified Delphi method to achieve consensus, as previously described7,8. The broad objective of ADQI 16 was to produce expert-based statements and a summary of current knowledge pertaining to the definition and management of AKD for use by clinicians and researchers according to ADQI's professional judgment and to identify evidence gaps to establish research priorities. Conference participants were divided into four work groups, which were tasked with formulating strategies for the initial workup and management of AKD and renal recovery in four different areas: Group 1 was tasked with developing recommendations for defining persistent AKI and AKD. Group 2 was tasked with developing definitions and staging for AKD. Group 3 developed recommendations for the management of patients with AKI and/or AKD requiring renal replacement therapy (RRT). Group 4 was tasked with developing recommendations for the management of medications among patients with AKD. Members of the work groups performed reviews of the literature in a systematic manner and developed a consensus of opinion, backed by evidence where possible, to distil the available literature and articulate a research agenda to address important unanswered questions. In addition, the members were asked to note the level of evidence for all consensus statements using the Oxford Centre for Evidence-based Medicine Levels of Evidence9. All of the individual workgroups iteratively presented their output to conference participants and the final product was then assessed and aggregated in a session attended by all attendees, who formally voted and approved the consensus recommendations. Discussion of the use of peritoneal dialysis as an option for treating AKI was excluded as this approach is typically used in austere conditions and special circumstances (for example, in small children).
Persistent AKI
Transient versus persistent AKI
Various studies, generally limited by the patient populations selected and the use of serum creatinine changes to assess renal function, have applied different thresholds for the duration of AKI episodes and functional renal recovery to discriminate transient from persistent AKI (see Supplementary information S1 (table)). Regardless of disease severity, these studies demonstrate that complete and sustained reversal of an AKI episode within 48–72 h of its onset is associated with better outcomes than longer durations of AKI10,11,12,13,14,15. Based on the available data and expert opinion, the workgroup proposes using 48 h to define rapid reversal of AKI (Box 1). The rationale for selecting 48 h rather than 72 h to define rapid reversal is to better identify high-risk patients for whom additional workup and evaluation might be warranted. Although previous studies have relied primarily on serum creatinine to identify AKI, the workgroup recommends also using urine output criteria as recommended by KDIGO5. The importance of urine output criteria in defining persistent AKI was confirmed in a 2015 study of 32,045 critically ill patients, which demonstrated that short-term and long-term risk of death or RRT is greatest for patients who meet both the serum creatinine and urine output criteria for AKI and experience these abnormalities for longer than 3 days12.
For AKI that has reversed, it is unknown when sustained reversal can be considered to have occurred. Although the duration of sustained reversal might be different for rapidly reversing and persistent AKI we propose a minimum of 48 h as being necessary to separate two distinct episodes of AKI. After sustained reversal has occurred, a second episode of AKI should be considered independently of the first, with new investigations to exclude potentially new reversible causes or contributing factors. This time period of 48 h to separate distinct AKI episodes is arbitrary and will require further study and validation.
Identification of persistent AKI
Early identification of persistent AKI is important in order to initiate an extended evaluation and management protocol to avoid further kidney damage and associated mortality16. An array of tools including clinical scoring systems, imaging approaches, and biomarkers must be developed to identify patients at risk of persistent AKI. A 2016 study of nearly 17,000 patients demonstrated that persistence of AKI and a stuttering versus prompt recovery pattern are linked to morbidity and mortality17. Importantly, these data suggest that interventions that alter the recovery pattern of AKI have the potential to improve patient outcomes. As most patients with severe sepsis — an important cause of AKI — present to the hospital with ongoing AKI, approaches to mitigate injury and enhance recovery from AKI should be areas of immediate focus18. However, clinical risk scores for persistent AKI have not been validated for general use and the risk factors that contribute to persistent AKI, AKD, and delayed recovery among hospitalized patients are not known. Several studies have identified clinical risk scores, biomarkers, imaging, and functional tests to differentiate rapid reversal of AKI from persisting AKI19,20,21,22,23,24,25,26,27 (see Supplementary information S2 (table)). In the opinion of the ADQI workgroup, these tools would likely work well together and are a recommended area of future research (Box 2).
Management of persistent AKI
Persistent AKI occurs in a subset of patients with AKI17; given the poor outcomes associated with persistent AKI, the ADQI workgroup recommends the presence of persistent AKI as a wake-up call to initiate further assessment and evaluation of treatment options. When a diagnosis of persistent AKI is made, the clinician should reassess the patient carefully and reconsider treatment options. First, the aetiology of the AKI should be considered. In most cases this aetiology is multifactorial, and it will occur secondary to another disease (for example, sepsis or shock) and notably can occur in early, middle, or late phases of the patient's hospital stay2,28. A diagnosis of persistent AKI should prompt re-evaluation of the possible causes of AKI, and correction of the underlying cause(s) when possible. Identification of potential causes of AKI might require additional tests such as evaluation of urine sediment, proteinuria, biomarker assessment and/or imaging. In select circumstances, consultation of other specialties might be needed to help diagnose rare causes of AKI (for example, caused by tumour lysis syndrome, thrombotic thrombocytopenic purpura and cholesterol embolization syndrome).
Approaches to assess renal function. Current approaches to measure glomerular filtration rate (GFR) with inulin, 51Cr-EDTA, or iohexol are time-consuming and laborious, and are therefore unsuitable for use in patients in intensive care. Equations for estimated GFR (eGFR), such as the modification of diet in renal disease (MDRD) or chronic kidney disease epidemiology collaboration (CKD–EPI) equations, were validated in patients with CKD. These equations can be used in the outpatient setting but not in the ICU setting, because they require serum creatinine to be in 'steady-state' (Ref. 29). As an alternative, short timed urine creatinine clearance (CCr) can be used to estimate GFR. However, several limitations exist to the use of CCr in ICU patients. For instance, CCr will often overestimate GFR, especially in patients with AKI, as creatinine is also excreted in the tubules, and establishing steady state conditions is often not possible30,31,32.
Some additional approaches to estimate GFR deserve further exploration. The Jelliffe equation for unstable kidney function, which is calculated on the basis of the volume of distribution and creatinine kinetics rather than steady state parameters such as body weight or age, correlated well with CCr in a small study of 12 patients in the ICU33,34. The kinetic eGFR, which similarly to the Jelliffe equation, estimates GFR on the basis of the creatinine kinetics has shown promise in renal transplant recipients, but should be validated in other cohorts such as hospitalized patients with native kidneys35. Iohexol clearance has been used in critically ill patients but as mentioned above, is laborious and time consuming36,37. Finally, fibreoptic ratiometric fluorescence analysis has shown promise for the measurement of GFR in large animals but awaits validation in human clinical settings38. Given that patients who have persistent AKI have worse outcomes than those of patients who recover from AKI, the ability to predict the clinical course of AKI with use of biomarkers of tubular or glomerular injury might help to differentiate these patients from those who will recover and enable prediction of outcomes. We therefore recommend that further research relating to biomarkers and renal functional tests should focus on this cohort of patients with AKI (Box 2).
From a treatment standpoint, patients with persistent AKI should be re-assessed on a daily basis bearing in mind at least two key considerations. First, the ongoing volume needs of the patient and risk of volume overload, and second, an assessment of the necessity of nephrotoxic medications and their appropriate dosing, balancing the risk of AKI and the benefits of each individual drug for the patient. Optimization of haemodynamic and volume status are important for the resolution of AKI, and these parameters should be evaluated closely39.
Baseline creatinine assessment
The best method for assessing baseline creatinine level can be uncertain given the inherent biologic variation in serum creatinine and the fact that serum creatinine measurements are made only when clinically indicated40. Although no approach to the assessment of baseline creatinine is perfect, the goal should be to reduce bias in anchoring the definition of AKD and its recovery. Towards that end, the use of known creatinine values is superior to imputation41. For patients in whom one or more pre-morbid serum creatinine values are available but show significant fluctuation, the choice of the serum creatinine measurement that best reflects the most appropriate baseline value may require adjudication by an expert clinician. In a large dataset, the mean serum creatinine value assessed 7–365 days before admission closely approximated expert clinical adjudication of baseline creatinine level41. Differences in misclassification were, however, modest compared with other available creatinine values, including the measurement taken at the time closest to hospital admission compared to the previous 7–365 days, which might be preferable in certain populations such as in patients undergoing elective surgery, those with progressive CKD, or those with a recent history of AKI.
For patients in whom no pre-morbid serum creatinine values are available, various methods for estimating these values have been studied, including imputing previous values41,42,43. Knowing the strengths and limitations of each approach is key to interpreting future study findings. For example, the accuracy of estimating a creatinine value using back-calculation from an eGFR of 75 ml/min/1.73 m2 has been previously studied44. This approach is likely the most sensitive for detecting AKI among patients with no premorbid serum creatinine value and is anticipated to work well in populations with largely preserved kidney function. In populations with a high prevalence of risk factors for CKD, however, this method might overestimate the incidence and severity of AKI and, therefore, AKD.
Acute kidney disease
AKI is a risk factor for the future loss of kidney function, cardiovascular disease, and death11,45,46,47,48,49,50. Defining optimal follow-up care for this high-risk population is therefore essential, especially during the transition of care beyond the acute care setting when recovery from AKI and its underlying precipitants might be ongoing. In this section, we examine surveillance approaches and interventions for survivors of AKI from hospital discharge to 90 days after the onset of renal dysfunction, identify knowledge gaps in the current understanding of AKD and its trajectories, and suggest approaches to address these knowledge gaps with the aim of defining approaches for the care of these individuals. We also propose an operational framework for AKD, which integrates with the KDIGO AKI classification scheme to characterize changes in kidney function or injury that do not meet strict criteria for AKI or CKD, including important patient-centred outcomes such as renal recovery. Here, we present three key concepts with regard to the follow-up of patients with AKD and a series of consensus statements developed through literature review and agreement within the ADQI workgroup.
Definition of AKD
AKD is defined as a condition in which AKI stage 1 or greater, as defined by KDIGO, is present ≥7 days after an AKI initiating event. AKD that persists beyond 90 days is considered to be CKD6 (Box 3).
An AKI initiating event can usually be identified but is not required to diagnose AKD. Typical scenarios in which patients may present with AKD include instances in which AKI is observed and the patient remains in KDIGO stage 1 or greater after 7 days; instances in which an episode of AKI was not observed (for example, in patients with community- acquired AKI18), but inferred by the persistence of kidney disease beyond 7 days (for patients without a known baseline creatinine value, clinical adjudication of AKD versus CKD might be required); instances in which subacute AKI, documented by either histology, imaging, proven biomarkers, or a relevant exposure (such as to a nephrotoxin), does not meet criteria for AKI or CKD; and instances in which AKI is observed, partially improves and then progresses after 7 days17 (Figs 2,3).
AKD follows on from acute kidney injury (AKI) in those patients who do not fully recover within 7 days. The trajectory of AKD can take many forms largely depending on the severity of the initial AKI episode. Here, a series of hypothetical scenarios representing typical trajectories of the AKI–AKD continuum are depicted. Stage 3 AKI might slowly improve to stage 2 AKI and then progress to AKD (1). Stage 1 AKI might progress to stage 3, then improve rapidly to stage 1 AKI before progressing to stage 1 AKD (2). An episode of persistent AKI (>48 h) might be followed by a period of sustained reversal(*), then a second episode of AKI (‡) leading to AKD (3). Stage 2 AKI might rapidly reverse (4). Subacute AKD might occur wherein the first 7 days are marked with slowly worsening renal function that does not technically meet the criteria for AKI, and progress to Stage 3 AKD (5). This trajectory can be seen in patients treated with chronic nephrotoxic medications (for example, with aminoglycosides). Modified from Acute Dialysis Quality Initiative 16; www.adqi.org.
In 2012, the KDIGO AKI workgroup proposed the term AKD to define any acute condition that impacts kidney function including AKI, eGFR <60 ml/min/1.73 m2, a decrease in GFR by >35%, an increase in serum creatinine of >50%, or any kidney damage lasting <3 months5. The goal of this operational definition was to “provide an integrated clinical approach to patients with acute abnormalities of kidney function and structure” and help to unify the more established concepts of AKI and CKD. Herein, the ADQI workgroup propose a new definition of AKD to further refine these criteria; we have also added a staging system. Our conceptual framework of AKD attempts to capture the entire spectrum of acute and subacute disease (including AKI), beginning with the initiation of injury (Fig. 2), as recognized using either conventional or novel markers of injury, its evolution, and kidney end points up to 90 days after the onset of injury. This updated AKD classification has two main features. First, it recognizes an important population of patients with evolving kidney disease who might not fulfil strict criteria for AKI (or CKD), such as those with kidney disease the onset of which is uncertain or subacute. Second, it highlights that the process of AKD can include AKI and extends beyond mere detection and staging of disease through the process of recovery or worsening until criteria for incident or worsening CKD are reached.
Various hypothetical trajectories of AKI exist (Fig. 3); however, important knowledge gaps need to be addressed before AKD terminology can be meaningfully used in clinical practice51,52 (Box 4). To date, only one study has attempted to characterize AKD using kidney biopsy samples53. Although the study findings suggest that the distribution of aetiologies that contribute to AKD without AKI might differ from those that contribute to AKI alone, the population studied (273 patients in China) was selected for clinical indications for biopsy and is likely not reflective of the broader population of patients with AKD. Further work is warranted to delineate the epidemiology of AKD, including differences in the predictors, course, and outcomes relative to AKI. Few data exist on characterizing the phases of AKD, including the processes by which patients recover or progress to CKD, the evolving risk experienced by AKD survivors, and the processes of care experienced.
For the purposes of our recommendations, AKD is conceptualized not as pre-CKD but rather, as post-AKI. This distinction has important implications for the diagnosis, care and follow-up of affected patients, including the notion that AKD might exist even in the absence of standard clinical evidence (Figs 2,4).
AKI stages map directly to the new proposed AKD stages. In addition, patients with AKD can progress to CKD. Stage 0 AKD represents partial recovery from AKI. Stage 0C includes patients for whom serum creatinine levels are higher than baseline but within 1.5 times baseline levels. Stage 0B includes patients whose serum creatinine has returned to baseline levels, but who still have evidence of ongoing kidney damage, injury, or loss of renal reserve. Stage 0A includes those patients who have had an episode of AKI and retain a risk of long-term events without structural or damage markers for AKD. Patients whose serum creatinine level has not returned to baseline and who have ongoing evidence of kidney damage and/or injury are termed stage 0B/C.
The ideal definition for recovery should quantify lost pre-existing kidney function as well as current residual kidney function and reserve, identify when recovery is complete, and provide prognostic information (Box 3). Intrinsic to the concept of AKD is that acute loss of kidney function or damage extends beyond diagnosis and staging of AKI and highlights additional points of potential intervention from the onset of injury through to the more convalescent phase of disease that could modify long-term outcomes. No standardized definition of recovery from AKI or AKD exists, and only a few studies have evaluated the kinetics or trajectory of recovery from either AKI or AKD among patients not on dialysis (see Supplementary information S3 (table)). Although these studies have used varying time frames and thresholds of serum creatinine level to define recovery, the results generally show a graded association between recovery and future risk of mortality, loss of kidney function, and other morbidities.
Other potential measures of recovery
AKD and recovery from AKD are currently assessed using filtration markers, such as serum creatinine. This approach has limitations, however, and loss of muscle mass, changes in volume of distribution, changes in renal reserve, and hyperfiltration can confound the assessment of functional recovery54,55,56,57,58,59,60. The limitations of using serum creatinine to assess recovery are supported by observational data indicating that AKI is associated with an increased risk of CKD, even when accompanied by an apparent complete return of serum creatinine to baseline levels61,62.
Alternative or complementary measures of kidney function, including other filtration markers such as cystatin C and timed urine clearance measurements, could hold promise for improved phenotyping of functional recovery from AKD but require further validation before recommending their routine adoption into clinical practice35,63,64,65,66. Methods to assess glomerular functional reserve (for example, by assessing the effect of a protein load on GFR) or tubular functional reserve (for example, through furosemide stress testing or the administration of intravenous creatinine) have also been developed in the CKD setting but have yet to be applied to patients with AKD67,68. Interestingly, serum creatinine level has been the standard approach to the assessment of renal function for decades, but intravenously administered creatinine fails to increase GFR in humans, regardless of renal function68. Intravenous creatinine does, however, significantly increase creatinine clearance68, demonstrating that glomerular and tubular reserve do not necessarily correlate and suggesting that patients with CKD can maintain some preservation of glomerular renal reserve but fail to show any measurable tubular reserve68,69,70,71. On the basis of these findings, assessments of glomerular and tubular reserve are likely to assess different facets of kidney disease. Several studies have also examined the use of next-generation biomarkers of tubular injury and furosemide stress testing to predict recovery from AKI72,73. As many of these markers reflect ongoing tubular injury, most studies have focused on their ability to indicate the likelihood of recovery during early or peak AKI in select groups of patients (see Supplementary information S4 (table)). Further work is needed to determine the utility of these biomarkers in informing clinical decision-making.
A framework to classify AKD and recovery
A useful classification of recovery from AKD would quantify the extent to which kidney function was lost, indicate when repair is complete and damage is no longer occurring, provide a measure of a patient's current kidney function and reserve, and provide prognostic information. A scheme that aligns with and integrates the KDIGO categories for AKI and provides a simple and translatable framework for ascertaining transition points for outcomes during AKD and at the end of 90 days would be ideal. Accordingly, we propose to map the KDIGO AKI staging categories to the staging of AKD for the purpose of defining the severity of AKD and to offer a framework for kidney-specific outcomes across a 90-day timeline (Fig. 4). In this conceptual framework, improvements in kidney function and/or resolution in kidney damage would be staged by an improvement (decrease) in AKD stage (for example, a shift from stage 3 AKD to stage 2 or lower). We recognize that specific thresholds to define 'recovery' remain to be defined, in particular in selected populations such as survivors of critical illness or among patients who no longer fulfil criteria for AKI or AKD stage 1 but whose serum creatinine level has not yet returned to baseline62,74. In this framework, we propose a 'stage 0', with A, B, and C subgroups (Table 1). Stage 0C includes patients for whom serum creatinine levels are higher than baseline but within 1.5 times baseline levels. Population studies suggest that these patients who achieve a recovery serum creatinine level that remains above 115% of baseline levels still carry a mortality risk74. Thus, these patients with AKD might require further follow-up and could be candidates for future therapeutic intervention. Stage 0B includes patients whose serum creatinine has returned to their baseline level after an episode of AKI, but still have evidence of ongoing kidney damage. The diagnosis of this ongoing damage for most patients will likely be in the form of new-onset proteinuria, worsened proteinuria from baseline, new-onset hypertension, or worsening hypertension. In addition to proteinuria and hypertension, evidence of ongoing kidney disease might be assessed through use of biomarkers or imaging studies. Stage 0B also includes patients for whom serum creatinine level has returned to baseline after an episode of AKI with no evidence of ongoing kidney damage, but who have experienced a loss of renal reserve. One example of this scenario would be a patient who has undergone a nephrectomy, whereby the contralateral kidney might adapt to the loss of renal mass, but a significant portion of renal reserve has nonetheless been lost. The assessment of renal reserve can be assessed by both glomerular and tubular stress testing75. Patients in whom serum creatinine levels fail to return to baseline and have evidence of ongoing injury would be classified as having Stage 0B/C. As the study of AKD is nascent, future research should carefully assess the risk of future events associated with these AKD stages (Box 4). In addition, the thresholds of the various biomarkers, imaging outputs, and/or renal reserve that define 'full recovery' versus ongoing risk is not known and will require further investigation. Stage 0A encompasses patients who have no evidence of damage or functional loss following an AKI episode and represents clinical recovery. These patients may nonetheless be vulnerable to further kidney damage and other adverse events. As has been shown previously, patients who have suffered an AKI event and 'recover' still carry a long-term increased risk of major adverse cardiac and kidney events49,76. Patients with AKD stage 0A might still require follow-up and could likely benefit from avoiding unnecessary nephrotoxic drugs. We hypothesize that this framework will enable the recognition and description of the dynamic nature of AKI and AKD beyond the initial diagnosis and staging of kidney injury, which will enable improved understanding of the natural course of the disease and ultimately facilitate the development of specific care pathways to guide surveillance, investigation and interventions, and align with care beyond 90 days. However, the accuracy and usefulness of these proposed stages in assessing kidney function and damage in patients with AKD requires further validation.
Finally, in keeping with the original conceptual framework for AKD as proposed by KDIGO, we recognize that AKI might not have always been diagnosed in a patient who appears to have an acute deterioration in renal function. In other words, the diagnosis of AKD may require inference of the existence of an episode of AKI. For example, consider a patient who is seen for an annual internist visit. The patient's serum creatinine is found to be twice the level observed the year before and they describe a severe 'flu-like' illness 2 months prior that lasted a week but eventually resolved without medical attention. We would suggest that treating this situation as a likely case of AKD — for example, by requesting that the patient avoids unnecessary nephrotoxins, requesting follow-up serum creatinine measurements, and screening for CKD risk factors — would be reasonable.
Follow-up care
As the evidence linking AKI with loss of kidney function45,46,47,77,78,79,80, hypertension81,82, cardiovascular disease49,50,83, and death46,83,84,85,86,87 accumulates, determining the optimal care for this growing population is critical. The American Society of Nephrology AKI Advisory Group has highlighted the transition of care as a potential opportunity to reduce the long-term impact of AKI88, and hence, AKD. However, a paucity of data exists to indicate which interventions can reduce morbidity and mortality in AKI/AKD survivors.
A first step in developing effective care strategies is to understand how care during follow-up associates with long-term outcomes. One element that has been examined is which physicians care for patients with AKI following hospital discharge. Studies indicate that most survivors of AKI are not cared for by nephrologists89,90,91,92. Although data derived largely from observational cohort studies suggest that referral to nephrology care is associated with improved survival93, causality remains to be proven and the elements of care that drive this potential benefit have not been identified. Identifying the driver(s) of beneficial outcomes is of critical relevance as rapid growth in the incidence of AKD means that most survivors will be cared for initially by primary care physicians.
One potential process of care that might confer benefit during follow-up is close monitoring of kidney function. Currently, a lack of evidence exists to guide the timing, frequency, and methods to evaluate kidney function among patients following an episode of AKI. Current KDIGO guidelines recommend that patients are evaluated “3 months after AKI for resolution, new onset, or worsening of pre-existing CKD”. Data from Medicare claims and the Veterans Affairs database indicate that only 50–69% of patients have a serum creatinine level measured within 3 months of an episode of AKI and that assessment of proteinuria occurs even more infrequently94,95. We recommend that the intensity of surveillance should be proportionate to the risk of future outcomes (Fig. 5). For example, patients who have more severe or persistent AKD11,96, those with premorbid conditions that increase the risk of future CKD progression (for example, those with evidence of pre-existing CKD, diabetes and/or proteinuria), and those with recurrent disease or non-recovery (for example, those with congestive heart failure, cirrhosis, and/or malignancy with or without chemotherapy) might achieve greater benefit from earlier or more frequent surveillance than patients with a lower risk of future CKD97,98. This hypothesis is supported by data showing that rates of re-hospitalization and recurrent AKI are high among patients with similar risk factors95,98,99,100,101,102,103,104.
The severity of AKD should determine the frequency and intensity of follow-up care. Patients with more severe AKD should receive nephrology follow-up if feasible. Key modifiers that should prompt more frequent follow-up and assessment of kidney function are the presence of pre-existing chronic kidney disease, congestive heart failure, cirrhosis, and/or malignancy. Modified from Acute Dialysis Quality Initiative 16; www.adqi.org.
We propose a conceptual 'layered' approach to the follow-up care of patients with AKD whereby the intensity of care rises in proportion to the risk of intermediate and long-term morbidity and mortality (Figs 5,6). Improving patient awareness of AKD and conditions or symptoms that might require evaluation of kidney function (such as oedema and volume-depleting illness), documenting that AKI and/or AKD has occurred particularly if moderate to severe or persistent, and processes of care including medication reconciliation to facilitate appropriate dosing and nephrotoxin avoidance, might help to alert future care providers to the risk of AKD, reduce the risk of adverse events including recurrent AKI, and potentially improve the probability of recovery88,105,106,107 (Box 4). Many of these elements of care are being examined and tested in prospective studies using 'post-AKI' care clinics that might better characterize which specific elements of care are most beneficial, which patients are most likely to benefit from different interventions, and the overall impact and cost of specialized care for patients with AKD108.
AKD can have various clinical courses and clinicians will be tasked with deciding when to change the dose, discontinue, and potentially re-introduce medications that are affected by kidney function and/or that are nephrotoxic. Assessment of renal function with use of biomarkers, glomerular filtration rate (GFR) measurement, and imaging should be performed across all stages of AKD as clinically indicated. Different possible scenarios are illustrated as follows: AKD begins to improve early in the clinical course (1); AKD is more entrenched, and kidney function improves only after a considerable decline in kidney function (2); AKD takes a severe course with kidney function recovery occurring after an extended decline in kidney function (3); severe AKD with progression to renal replacement therapy (4). Modified from Acute Dialysis Quality Initiative 16; www.adqi.org.
RRT recommendations for patients with CKD
The decision of when to initiate RRT is not standardized between countries, institutions or even between individual physicians within a group practice. Although initiation of RRT is usually associated with serious renal dysfunction corresponding to stage 3 AKI as defined by KDIGO, some instances exist in which RRT is initiated in the setting of non-severe renal dysfunction, for example, in the setting of electrolyte disturbances, fluid overload, toxic ingestions or poisoning. Thus, database studies that use RRT as an indicator of severe AKI might also include a small proportion of patients with less severe AKI. Nonetheless, the approach of using RRT as a marker of AKI severity has yielded consistent findings across more than 1 million patients assessed worldwide and remains an excellent surrogate for severe AKI in database studies5.
Assessing recovery from RRT dependence
Although current definitions for the recovery of patients from dialysis-dependent AKI are diverse and subjective, a unifying characteristic is sustained independence from RRT87,109,110. We suggest that organ (kidney) recovery in patients who have received acute RRT be defined as sustained independence from RRT for a minimum of 14 days (Box 5). This definition is not to say that independence from RRT cannot be assessed before 14 days and we appreciate that researchers might use various means to adjudicate independence from RRT before hospital discharge. However for individual patient care, we recommend close follow-up after hospital discharge to ensure that independence from RRT is indeed sustained.
In order to assess recovery from dialysis-dependent AKD, we suggest that laboratory and clinical evaluation after cessation of acute RRT should occur within 3 days (and no later than 7 days) after the last RRT session, and be followed by regular and frequent assessments thereafter. The interval for subsequent assessments should be based upon clinical judgment. We suggest that issues such as maintenance of dialysis access, medication reconciliation and evaluation of the appropriateness of medications and their dosing should be addressed at each clinical assessment. The patient's outpatient record should clearly state that the patient had RRT-requiring AKI and include a plan for outpatient care that includes measurement and documentation of kidney function. Furthermore, continued follow-up with a nephrologist is recommended.
For patients who are discharged while still receiving RRT, frequent review and documentation of kidney function should occur to assess the continued need for RRT. At a minimum, these reviews should include a weekly assessment of serial pre-dialysis serum creatinine values and regular assessment of residual kidney function using a 24 h urine collection to assess volume of urine output as well as creatinine and urea clearance. Careful consideration should be given to the temporary acute vascular access site, with avoidance of subclavian veins and the internal jugular vein on the side of a future potential arteriovenous fistula. Importantly, if the patient is discharged from the hospital to a chronic dialysis facility, the treating team should be informed that a personalized approach that maximizes the likelihood of renal recovery should be utilized. Specifically, avoidance of excessive fluid removal and hypotension are critical to prevent re-injury to the kidney and to enhance the likelihood of renal recovery.
We contend that the therapeutic goal for patients recovering from an episode of RRT-requiring AKI should be recovery of functional status to pre-morbid levels. Assessment of kidney function in patients who received RRT and recovered to RRT independence must take into account loss of muscle mass and its impact on standard markers of GFR such as serum creatinine29. The use of alternative markers of GFR that are not sensitive to muscle mass (for example, cystatin C) or the direct quantification of GFR (with iohexol clearance, for instance), should be considered in selected cases37.
Predicting outcomes
We suggest that novel biomarkers and approaches to the direct measurement of GFR might be valuable for the evaluation of kidney recovery among patients receiving RRT (Boxes 5,6). Pending the development of such tools, the utility of available markers, such as urine output, timed creatinine and/or urea clearances, to aid the prediction of successful RRT cessation should be studied further.
Data on the effect of patient characteristics on outcomes and on how to use these factors to influence decision making are currently limited (Box 5). Observational studies have identified several risk factors for non-recovery48,111,112,113,114,115,116,117 (see Supplementary information S5 (table)). Novel modalities that might enhance the prediction of non-recovery, including urine and plasma biomarkers, histopathologic markers on kidney biopsy specimens and imaging tools, should be carefully studied (Box 6). Regardless of the markers that are chosen, all assessments for non-recovery of kidney function must be analysed, while accounting for the competing risk of death.
It is possible that the operational characteristics of RRT might influence renal and patient recovery. Only limited high quality data exist on the effects of operational characteristics of RRT on recovery of kidney function among patients with RRT-dependent AKD87,118,119,120,121,122,123 (Box 5; Table 2). Findings from a single randomized trial suggest that utilization of strict guidelines to improve therapy tolerance and metabolic control renders intermittent RRT comparable to continuous RRT123. We further acknowledge that numerous other factors involved in the process of care might influence renal recovery among patients with dialysis-dependent AKD73,124 (see Supplementary information S6 (table)).
Drug dosing during AKD
The selection and dosing of drugs in patients with AKD requires multiple and dynamic assessments, in which understanding of the phases of AKD, including the timing of the initial insult, and the likelihood of AKD reversal, persistence, recovery and/or progression to CKD should prompt clinical review of prescribed medications (Box 7; Figs 5,6). Assessment of the medication regimen comprises several components (Table 3). The disposition and effects of drugs administered to patients with AKD are modulated by a number of factors, including changes in drug clearance (which is dependent on glomerular and tubular function, and non-renal drug metabolism), and altered pharmacokinetic parameters due to decreased kidney function (for example, volume overload and metabolic acidosis). In addition, the mechanism of nephrotoxicity, whether from direct tubular toxicity (such as caused by aminoglycosides), reno- vasoconstriction (such as caused by NSAIDs and radiocontrast media), interstitial nephritis (such as caused by NSAIDs and β-lactams) or crystallization (as caused by acyclovir), should be considered in the context of the functional phase of AKD. For example, withholding NSAIDs might make sense while a patient is in the persistent or recovery phase of AKD whereas careful dosing and monitoring of aminoglycosides to prevent re-injury in the recovery phase of AKI might be warranted.
Factors that must be taken into account when selecting a treatment regimen include considerations as to the mode of drug excretion (renal versus non-renal); the potential for nephrotoxicity; the effect of AKD on drug metabolites and/or the effect of AKD on the non-renal metabolism of drugs; the strength of indications and/or urgency for their use; and the availability of suitable alternatives (Box 7).
The relevance of each of these considerations for a particular drug is likely to vary, and once again, should be taken in the context of the AKD stage, with reassessment as patients transition from one AKD stage to another, including the identification of patients who are at risk of nephrotoxicity before exposure to the toxic agent. For instance, avoidance of nephrotoxic medications such as aminoglycosides or NSAIDs in a patient at risk of AKI (such as in patients with CKD, a previous history of AKI, or in those who are already taking multiple nephrotoxic medications), who is admitted to the ICU would make sense, unless that medication is clearly superior in terms of efficacy and no suitable alternative exists. Early in the AKI course when GFR is starting to fall, a systematic reassessment of drug dosing, surveillance of drug concentrations when available, and avoidance of nephrotoxic medications or drugs with a renovascular effect should be undertaken. Various factors relating to drug avoidance or the reintroduction of drugs in various AKD stages need to be considered (Box 8; Tables 3,4). Below, we discuss considerations relevant to angiotensin-converting-enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs), two nearly ubiquitously prescribed medications with renovascular effects.
ACE inhibitors and ARBs
At present the armamentarium available for facilitating the transition from AKI to recovery is limited and the decision to restrict therapies might reflect the nephrotoxic potential of some drugs. Perhaps the most relevant examples are ACE inhibitors and ARBs, which are associated with functional AKI, particularly in the setting of acute hypovolaemia5,125,126,127. These agents are frequently prescribed, particularly in the elderly128. A 2013 study from the UK that used routinely collected national hospital administrative data showed that a 16% increase in ACE inhibitor and ARB prescribing between 2007 and 2011 corresponded with a 50% increase in the number of hospital admissions complicated by AKI in the same time period129. Although ACE inhibitors and ARBs have benefits, the risk–benefit ratio in patients with AKD might not reflect that observed in routine clinical practice. Whether stopping these drugs during periods of AKI and/or AKD results in better outcomes, or how often and at what stage they should be restarted following recovery from AKI and/or AKD is not known.
Despite recommendations that ACE inhibitors and ARBs are routinely stopped during any intercurrent illness130, sparse evidence exists to support these recommendations131. Two studies in which these agents were not re-started in patients after surgery demonstrated an increase in 30 day mortality, possibly from hypertensive rebound leading to acute cardiac decompensation132,133. Re-introduction of ACE inhibitors and ARBs in acute illness is usually considered when GFR has stabilized and volume status is optimized. Hypotension and decreased filtration fraction are recognized as common adverse effects associated with ACE inhibitor and ARB use that can cause or exacerbate AKI, and the risk–benefit ratio for their use in patients with AKD must be carefully considered and therapy personalized according to the individual risks of the patient. Although chronic tolerance to reversible decrements in filtration fraction and GFR caused by ACE inhibitors and ARBs might be desirable in patients with chronic heart failure and CKD, such effects might not be tolerable and are without proven benefit in patients with AKD. Similarly, despite a significant risk of potential therapeutic failure caused by under dosing or avoidance of these most effective drugs in patients with AKD (particularly in the recovery phase), such therapeutic failure is rarely recorded134.
Effect of AKI and AKD on drug metabolism
The effects of CKD on drug metabolism and subsequent dosing regimens are well established but little is known about the effects of AKI or AKD on drug metabolism135. Extrapolation of data from patients with CKD is not ideal given that the time course of disease progression is different. Organ crosstalk, particularly involving the liver and the kidney, can influence drug metabolism135, which could reflect the impact of AKI on hepatic blood flow, the consequences of metabolic acidosis or changes in protein binding136 on drug distribution, and the increasingly recognized effects of AKI on cytochrome P450 activity; overall, the impact of AKI on hepatic drug metabolism seems to be clinically relevant. Impairment of cytochrome P450 activity, as well as effects on drug transporters, could also account for some of the pharmacodynamic effects of AKI.
Nephrotoxin management during AKD
In developed countries, drugs account for 20% of community-acquired AKI episodes that result in hospitalization137,138. Drug-associated AKI (DA-AKI) occurs in approximately 25% of critically ill patients, making drugs a common cause of AKI in the ICU28,139,140. The consequences of DA-AKI are severe, with rates of dialysis dependence and/or risk of mortality similar to those of AKI resulting from other aetiologies (40–50%)140. Early reversal of AKI from other aetiologies leads to improved survival compared to that of patients with persistent AKI or new-onset AKI, suggesting that early reversal of DA-AKI might also be associated with improved outcomes14.
Evaluation of nephrotoxins as a plausible cause of AKI is the first consideration in the management of medications for patients with AKI. Determining nephrotoxic causality involves assessment of the temporal sequence between administration and the onset of injury, other possible causes, response to the removal of a drug, and in some cases the effects of restarting the drug141. In all phases of AKD, selection of a less nephrotoxic drug and/or avoidance of a nephrotoxin should be the goal. This approach is supported by the fact that each nephrotoxin administration presents a 53% greater odds of developing AKI142, and is compounded when patients receive more than one nephrotoxin143. Combining nephrotoxins can result in pharmacodynamic drug interactions, such as the 'triple whammy' of NSAIDs, diuretics and ACE inhibitors or ARBs125. In the non-ICU setting, escalating the burden of nephrotoxic medications from two to three medications more than doubles the risk of developing AKI, and 25% of non-critically ill patients who receive three or more nephrotoxins develop AKI88,144. Pharmacokinetic drug interactions arising from the administration of some macrolide antibiotics (such as clarithromycin or erythromycin) together with a 3-hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA) reductase inhibitor (statin) result in a greater number of hospitalizations for AKI from rhabdomyolysis, than those arising from administration of azithromycin (a macrolide that does not powerfully inhibit cytochrome p450 enzyme CYP 3A4 and therefore impair statin clearance)145.
An evaluation of the appropriate timing to administer a drug assumes that a nephrotoxin is essential for the patient. The treatment of an infection with an antibiotic that is necessary for survival should begin immediately, and might prevent or ameliorate AKI. Determining whether nephrotoxins are a possible cause or contributor to AKI requires thorough evaluation146,147. The persistent phase of AKD necessitates the continued consideration of nephrotoxin avoidance. During the recovery phase of AKD, caution should still be applied to nephrotoxin initiation, to prevent re-injury.
A general statement cannot be made about a functional threshold at which to avoid or discontinue nephrotoxins. The recommendations provided in package inserts or guidelines specific to a drug or drug class might offer guidance. For example, combination trimethoprim and sulfamethoxazole treatment is not recommended if creatinine clearance is <15 ml/min. The revised Beers criteria for potentially inappropriate medication use in older adults indicate moderate evidence to support NSAID avoidance in elderly patients with creatinine clearance <30 ml/min (Ref. 148).
Detection and management of nephrotoxins at the initiation of AKI146,147,149,150,151 and during CKD152,153 have been well described but fewer data exist for nephrotoxin management during AKD. Two groups reported together on a multicomponent medical management approach to patients following an episode of AKI, including the management of drugs. Patients are educated to avoid taking NSAIDs (or any new medications) without consulting their nephrologist, and to use ACE inhibitors, decongestants, antivirals, antibiotics and herbal products with caution108.
Conclusions
AKD represents an important transition period for patients who have suffered an episode of AKI. Considerable advances have been made in our collective understanding of AKI and CKD; however, the relationship between these two conditions is vitally important because these two syndromes are interconnected. One of the most significant risk factors for AKI is pre-existing CKD, and AKI is a significant risk factor for the development of CKD as well as the progression of pre-existing CKD4. A critical period of vulnerability for patients who develop AKI is in the immediate period following development of AKI — a period previously labelled AKD. In this manuscript, the ADQI workgroup offers a series of proposed definitions and recommendations that aim to increase awareness of AKD and encourage research that will guide future understanding of the epidemiology, mechanisms and management of AKD.
As we have proposed new definitions, provided guidance for clinical practice and put forth a large agenda for future research, it will be incumbent on the AKI clinical research community to test our recommendations. Importantly, as many patients with AKI present to the hospital with ongoing AKI, a critical intervention point to facilitate recovery and minimize continuing damage will occur during AKD. As such, the ADQI 16 workgroup advocates a sense of urgency behind both the adoption of our clinical recommendations and the execution of the proposed research agenda.
References
Lewington, A. J., Cerda, J. & Mehta, R. L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 84, 457–467 (2013).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Tao Li, P. K., Burdmann, E. A. & Mehta, R. L. Acute kidney injury: global health alert. Int. J. Organ Transplant. Med. 4, 1–8 (2013).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66 (2014).
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2, 1–138 (2012).
Levey, A. S. et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 67, 2089–2100 (2005).
Bagshaw, S. M., Goldstein, S. L., Ronco, C., Kellum, J. A. & Group, A. C. Acute kidney injury in the era of big data: the 15(th) Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Can. J. Kidney Health Dis. 3, 5 (2016).
Kellum, J. A., Bellomo, R. & Ronco, C. Acute Dialysis Quality Initiative (ADQI): methodology. Int. J. Artif. Organs 31, 90–93 (2008).
Centre for Evidence-Based Medicine. Oxford Centre for Evidence-based Medicine — Levels of Evidence (March 2009). CEBM http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (2009).
Brown, J. R., Kramer, R. S., Coca, S. G. & Parikh, C. R. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann. Thorac. Surg. 90, 1142–1148 (2010).
Coca, S. G. et al. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 78, 926–933 (2010).
Kellum, J. A. et al. Classifying AKI by urine output versus serum creatinine level. J. Am. Soc. Nephrol. 26, 2231–2238 (2015).
Perinel, S. et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit. Care Med. 43, e269–e275 (2015).
Sood, M. M. et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J. Crit. Care 29, 711–717 (2014).
Uchino, S., Bellomo, R., Bagshaw, S. M. & Goldsmith, D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol. Dial. Transplant. 25, 1833–1839 (2010).
Korenkevych, D. et al. The pattern of longitudinal change in serum creatinine and 90-day mortality after major surgery. Ann. Surg. 263, 1219–1227 (2016).
Kellum, J. A. et al. Recovery after acute kidney injury. Am. J. Respir. Crit. Care Med. 65, 528–529 (2016).
Kellum, J. A. et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am. J. Respir. Crit. Care Med. 193, 281–287 (2016).
Brown, J. R. et al. Determinants of acute kidney injury duration after cardiac surgery: an externally validated tool. Ann. Thorac. Surg. 93, 570–576 (2012).
Darmon, M. et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 37, 68–76 (2011).
Schnell, D. et al. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock 38, 592–710 (2012).
Platt, J. F., Rubin, J. M. & Ellis, J. H. Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology 179, 419–423 (1991).
Dewitte, A. et al. Doppler resistive index to reflect regulation of renal vascular tone during sepsis and acute kidney injury. Crit. Care 16, R165 (2012).
Ninet, S. et al. Doppler-based renal resistive index for prediction of renal dysfunction reversibility: a systematic review and meta-analysis. J. Crit. Care 30, 629–635 (2015).
de Geus, H., Bakker, J., Lesaffre, E. & leNoble, J. Neutrophil gelatinase-associated lipocalin at ICU admission predict for acute kidney injury in adult patients. Am. J. Respir. Crit. Care Med. 183, 907–914 (2011).
Basu, R. K. et al. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin. J. Am. Soc. Nephrol. 9, 654–662 (2014).
Chawla, L. S. et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit. Care 17, R207 (2013).
Uchino, S. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818 (2005).
Carlier, M. et al. Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med. 41, 427–435 (2015).
Chrymko, M. M. & Schentag, J. J. Creatinine clearance predictions in acutely ill patients. Am. J. Hosp. Pharm. 38, 837–840 (1981).
Kwong, M. B., Tong, T. K., Mickell, J. J. & Chan, J. C. Lack of evidence that formula-derived creatinine clearance approximates glomerular filtration rate in pediatric intensive care population. Clin. Nephrol. 24, 285–288 (1985).
Hoste, E. A. et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol. Dial. Transplant. 20, 747–753 (2005).
Jelliffe, R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am. J. Nephrol. 22, 320–324 (2002).
Bouchard, J. et al. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol. Dial. Transplant. 25, 102–107 (2010).
Pianta, T. J., Endre, Z. H., Pickering, J. W., Buckley, N. A. & Peake, P. W. Kinetic estimation of GFR improves prediction of dialysis and recovery after kidney transplantation. PLoS ONE 10, e0125669 (2015).
Benz-de Bretagne, I. et al. New sampling strategy using a Bayesian approach to assess iohexol clearance in kidney transplant recipients. Ther. Drug Monit. 34, 289–297 (2012).
Dixon, J. J. et al. Validation of a continuous infusion of low dose Iohexol to measure glomerular filtration rate: randomised clinical trial. J. Transl Med. 13, 58 (2015).
Wang, E. et al. A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int. 81, 112–117 (2012).
Hoste, E. A. et al. Four phases of intravenous fluid therapy: a conceptual model. Br. J. Anaesth. 113, 740–747 (2014).
Siew, E. D. & Matheny, M. E. Choice of reference serum creatinine in defining acute kidney injury. Nephron 131, 107–112 (2015).
Siew, E. D. et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin. J. Am. Soc. Nephrol. 7, 712–719 (2012).
Siew, E. D. et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 77, 536–542 (2010).
Siew, E. D. Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin. J. Am. Soc. Nephrol. 8, 10–18 (2013).
Zavada, J. et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol. Dial. Transplant. 25, 3911–3918 (2010).
Amdur, R. L. et al. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 76, 1089–1097 (2009).
Hsu, C. Y. et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin. J. Am. Soc. Nephrol. 4, 891–898 (2009).
Wald, R. et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302, 1179–1185 (2009).
Wu, V. C. et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 80, 1222–1230 (2011).
Wu, V. C. et al. Long-term risk of coronary events after AKI. J. Am. Soc. Nephrol. 25, 595–605 (2014).
Monseu, M. et al. Acute kidney injury predicts major adverse outcomes in diabetes: synergic impact with low glomerular filtration rate and albuminuria. Diabetes Care 38, 2333–2340 (2015).
Palevsky, P. M. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 61, 649–672 (2013).
James, M. et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 61, 673–685 (2013).
Chu, R. et al. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin. J. Am. Soc. Nephrol. 9, 1175–1182 (2014).
Prowle, J. R. et al. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin. J. Am. Soc. Nephrol. 9, 1015–1023 (2014).
Liu, K. D. et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit. Care Med. 39, 2665–2671 (2011).
Doi, K. et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J. Am. Soc. Nephrol. 20, 1217–1221 (2009).
Moran, S. M. & Myers, B. D. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 27, 928–937 (1985).
Bosch, J. P., Lauer, A. & Glabman, S. Short-term protein loading in assessment of patients with renal disease. Am. J. Med. 77, 873–879 (1984).
Thomas, D. M., Coles, G. A. & Williams, J. D. What does the renal reserve mean? Kidney Int. 45, 411–416 (1994).
Graf, H., Stummvoll, H. K., Luger, A. & Prager, R. Effect of amino acid infusion on glomerular filtration rate. N. Engl. J. Med. 308, 159–160 (1983).
Bucaloiu, I. D. et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 81, 477–485 (2012).
Heung, M. et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 67, 742–752 (2016).
Endre, Z. H., Pickering, J. W. & Walker, R. J. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI). Am. J. Physiol. Renal Physiol. 301, F697–F707 (2011).
Chen, S. Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J. Am. Soc. Nephrol. 24, 877–888 (2013).
Pickering, J. W., Frampton, C. M., Walker, R. J., Shaw, G. M. & Endre, Z. H. Four hour creatinine clearance is better than plasma creatinine for monitoring renal function in critically ill patients. Crit. Care 16, R107 (2012).
Endre, Z. H. Recovery from acute kidney injury: the role of biomarkers. Nephron Clin. Pract. 127, 101–105 (2014).
Ronco, C. & Chawla, L. S. Glomerular and tubular kidney stress test: new tools for a deeper evaluation of kidney function. Nephron 134, 191–194 (2016).
Herrera, J., Avila, E., Marin, C. & Rodriguez-Iturbe, B. Impaired creatinine secretion after an intravenous creatinine load is an early characteristic of the nephropathy of sickle cell anaemia. Nephrol. Dial. Transplant. 17, 602–607 (2002).
Herrera, J. & Rodriguez-Iturbe, B. Stimulation of tubular secretion of creatinine in health and in conditions associated with reduced nephron mass. Evidence for a tubular functional reserve. Nephrol. Dial. Transplant. 13, 623–629 (1998).
Rodriguez-Iturbe, B., Herrera, J. & Garcia, R. Response to acute protein load in kidney donors and in apparently normal postacute glomerulonephritis patients: evidence for glomerular hyperfiltration. Lancet 2, 461–464 (1985).
Rodriguez-Iturbe, B., Herrera, J., Marin, C. & Manalich, R. Tubular stress test detects subclinical reduction in renal functioning mass. Kidney Int. 59, 1094–1102 (2001).
Srisawat, N. et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 80, 545–552 (2011).
van der Voort, P. H. et al. Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit. Care Med. 37, 533–538 (2009).
Pannu, N., James, M., Hemmelgarn, B., Klarenbach, S. & Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin. J. Am. Soc. Nephrol. 8, 194–202 (2013).
Chawla, L. S. & Ronco, C. Renal stress testing in the assessment of kidney disease. K. I. Rep. 1, 57–63 (2016).
Shiao, C. C. et al. Long-term remote organ consequences following acute kidney injury. Crit. Care 19, 438 (2015).
Ishani, A. et al. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 20, 223–228 (2009).
Ishani, A. et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch. Intern. Med. 171, 226–233 (2011).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
James, M. T. et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 78, 803–809 (2010).
Hsu, C. Y. et al. Elevated BP after AKI. J. Am. Soc. Nephrol. 27, 914–923 (2016).
Pechman, K. R. et al. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1358–R1363 (2009).
James, M. T. et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 123, 409–416 (2011).
Chawla, L. S. et al. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin. J. Am. Soc. Nephrol. 9, 448–456 (2014).
Pickering, J. W., James, M. T. & Palmer, S. C. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am. J. Kidney Dis. 65, 283–293 (2015).
Newsome, B. B. et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch. Intern. Med. 168, 609–616 (2008).
Palevsky, P. M. et al. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 359, 7–20 (2008).
Goldstein, S. L., Jaber, B. L., Faubel, S., Chawla, L. S. & Acute Kidney Injury Advisory Group of American Society of Nephrology. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin. J. Am. Soc. Nephrol. 8, 476–483 (2013).
Siew, E. D. et al. Outpatient nephrology referral rates after acute kidney injury. J. Am. Soc. Nephrol. 23, 305–312 (2012).
Kirwan, C. J. et al. Critically ill patients requiring acute renal replacement therapy are at an increased risk of long-term renal dysfunction, but rarely receive specialist nephrology follow-up. Nephron 129, 164–170 (2015).
Horkan, C. M. et al. The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study. Crit. Care Med. 43, 354–364 (2015).
Khan, I. H., Catto, G. R., Edward, N. & Macleod, A. M. Acute renal failure: factors influencing nephrology referral and outcome. QJM 90, 781–785 (1997).
Harel, Z. et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 83, 901–908 (2013).
Matheny, M. E. et al. Laboratory test surveillance following acute kidney injury. PLoS ONE 9, e103746 (2014).
United States Renal Data System Annual Data Report 2013. Acute Kidney Injury [online], https://www.usrds.org/2013/pdf/v1_ch6_13.pdf (2013).
Chawla, L. S., Amdur, R. L., Amodeo, S., Kimmel, P. L. & Palant, C. E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 79, 1361–1369 (2011).
Hickson, L. J. et al. Predictors of outpatient kidney function recovery among patients who initiate hemodialysis in the hospital. Am. J. Kidney Dis. 65, 592–602 (2015).
Siew, E. D. et al. Predictors of recurrent AKI. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2014121218 (2015).
Koulouridis, I., Price, L. L., Madias, N. E. & Jaber, B. L. Hospital-acquired acute kidney injury and hospital readmission: a cohort study. Am. J. Kidney Dis. 65, 275–282 (2015).
Brown, D. W., Giles, W. H. & Croft, J. B. White blood cell count: an independent predictor of coronary heart disease mortality among a national cohort. J. Clin. Epidemiol. 54, 316–322 (2001).
Kelleher, S. P., Robinette, J. B., Miller, F. & Conger, J. D. Effect of hemorrhagic reduction in blood pressure on recovery from acute renal failure. Kidney Int. 31, 725–730 (1987).
Solez, K., Morel-Maroger, L. & Sraer, J. D. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine 58, 362–376 (1979).
Xie, M. & Iqbal, S. Predictors for nephrology outpatient care and recurrence of acute kidney injury (AKI) after an in-hospital AKI episode. Hemodial. Int. 18 (Suppl. 1), S7–S12 (2014).
Huber, M. et al. Mortality and cost of acute and chronic kidney disease after vascular surgery. Ann. Vasc. Surg. 30, 72–81.e2 (2016).
Cox, Z. L. et al. Adverse drug events during AKI and its recovery. Clin. J. Am. Soc. Nephrol. 8, 1070–1078 (2013).
Coleman, E. A., Smith, J. D., Raha, D. & Min, S. J. Posthospital medication discrepancies: prevalence and contributing factors. Arch. Intern. Med. 165, 1842–1847 (2005).
Bell, C. M. et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA 306, 840–847 (2011).
Silver, S. A. et al. Improving care after acute kidney injury: a prospective time series study. Nephron 131, 43–50 (2015).
Duran, P. A. & Concepcion, L. A. Survival after acute kidney injury requiring dialysis: long-term follow up. Hemodial. Int. 18 (Suppl. 1), S1–S6 (2014).
RENAL Replacement Therapy Study Investigators et al. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 361, 1627–1638 (2009).
Ponce, D., Buffarah, M. B., Goes, C. & Balbi, A. Peritoneal dialysis in acute kidney injury: trends in the outcome across time periods. PLoS ONE 10, e0126436 (2015).
Heung, M. & Chawla, L. S. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr. Opin. Nephrol. Hypertens. 21, 628–634 (2012).
Bouchard, J. et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 76, 422–427 (2009).
Sharma, P. et al. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin. J. Am. Soc. Nephrol. 8, 1135–1142 (2013).
Gautam, S. C. et al. Predictors and outcomes of post-hospitalization dialysis dependent acute kidney injury. Nephron 131, 185–190 (2015).
Schiffl, H. & Fischer, R. Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol. Dial. Transplant. 23, 2235–2241 (2008).
Parker, R. A. et al. Prognosis of patients with acute renal failure requiring dialysis: results of a multicenter study. Am. J. Kidney Dis. 32, 432–443 (1998).
do Nascimento, G. V., Balbi, A. L., Ponce, D. & Abrao, J. M. Early initiation of dialysis: mortality and renal function recovery in acute kidney injury patients. J. Bras. Nefrol. 34, 337–342 (2012).
Schneider, A. G. et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 39, 987–997 (2013).
Wald, R. et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study. Crit. Care Med. 42, 868–877 (2014).
Jun, M. et al. Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Crit. Care Med. 42, 1756–1765 (2014).
Friedrich, J. O., Wald, R., Bagshaw, S. M., Burns, K. E. & Adhikari, N. K. Hemofiltration compared to hemodialysis for acute kidney injury: systematic review and meta-analysis. Crit. Care 16, R146 (2012).
Vinsonneau, C. et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet 368, 379–385 (2006).
Hu, S. L., Said, F. R., Epstein, D. & Lokeshwari, M. The impact of anemia on renal recovery and survival in acute kidney injury. Clin. Nephrol. 79, 221–228 (2013).
Lapi, F., Azoulay, L., Yin, H., Nessim, S. J. & Suissa, S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ 346, e8525 (2013).
Chao, C.-T. et al. Cumulative cardiovascular polypharmacy is associated with the risk of acute kidney injury in elderly patients. Medicine 94, e1251 (2015).
Ftouh, S., Thomas, M. & Acute Kidney Injury Guideline Development Group. Acute kidney injury: summary of NICE guidance. BMJ 347, f4930 (2013).
Smets, H. L., De Haes, J. F., De Swaef, A., Jorens, P. G. & Verpooten, G. A. Exposure of the elderly to potential nephrotoxic drug combinations in Belgium. Pharmacoepidemiol. Drug Saf. 17, 1014–1019 (2008).
Tomlinson, L. A. et al. ACE inhibitor and angiotensin receptor-II antagonist prescribing and hospital admissions with acute kidney injury: a longitudinal ecological study. PLoS ONE 8, e78465 (2013).
Onuigbo, M. A. Reno-prevention versus reno-protection: a critical re-appraisal of the evidence-base from the large RAAS blockade trials after ONTARGET — a call for more circumspection. QJM 102, 155–167 (2009).
Morden, A. et al. The risks and benefits of patients temporarily discontinuing medications in the event of an intercurrent illness: a systematic review protocol. Syst. Rev. 4, 139 (2015).
Mudumbai, C. et al. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J. Hosp. Med. 9, 289 (2014).
Lee, S. M., Takemoto, S. & Wallace, A. W. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 123, 288–306 (2015).
Cox, L. et al. Adverse drug events during AKI and its recovery. Clin. J. Am. Soc. Nephrol. 8, 1070 (2013).
Philips, B. J., Lane, K., Dixon, J. & Macphee, I. The effects of acute renal failure on drug metabolism. Expert Opin. Drug Metab. Toxicol. 10, 11–23 (2014).
Nicholson, J. P., Wolmarans, M. R. & Park, G. R. The role of albumin in critical illness. Br. J. Anaesth. 85, 599–610 (2000).
Kaufman, J., Dhakal, M., Patel, B. & Hamburger, R. Community-acquired acute renal failure. Am. J. Kidney Dis. 17, 191–198 (1991).
Wu, T. Y. et al. Ten-year trends in hospital admissions for adverse drug reactions in England 1999–2009. J. R. Soc. Med. 103, 239–250 (2010).
Brivet, F. G., Kleinknecht, D. J., Loirat, P. & Landais, P. J. Acute renal failure in intensive care units — causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit. Care Med. 24, 192–198 (1996).
Mehta, R. L. et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 66, 1613–1621 (2004).
Kane-Gill, S. L., Forsberg, E. A., Verrico, M. M. & Handler, S. M. Comparison of three pharmacovigilance algorithms in the ICU setting: a retrospective and prospective evaluation of ADRs. Drug Saf. 35, 645–653 (2012).
Cartin-Ceba, R. et al. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit. Care Res. Pract. 2012, 691013 (2012).
Rivosecchi, R., Dasta, J., Kellum, J. & Kane-Gill, S. 975: a unique application of the GRADE criteria: drug-combination associated Aki. Crit. Care Med. 43, 245 (2015).
Moffett, B. S. & Goldstein, S. L. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin. J. Am. Soc. Nephrol. 6, 856–863 (2011).
Patel, A. M. et al. Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study. Ann. Intern. Med. 158, 869–876 (2013).
Nachtigall, I. et al. Standard operating procedures for antibiotic therapy and the occurrence of acute kidney injury: a prospective, clinical, non-interventional, observational study. Crit. Care 18, R120 (2014).
Picard, W. et al. Propensity-based study of aminoglycoside nephrotoxicity in patients with severe sepsis or septic shock. Antimicrob. Agents Chemother. 58, 7468–7474 (2014).
By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 63, 2227–2246 (2015).
Kirkendall, E. S. et al. Development and performance of electronic acute kidney injury triggers to identify pediatric patients at risk for nephrotoxic medication-associated harm. Appl. Clin. Inform. 5, 313–333 (2014).
Goldstein, S. L. et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 132, e756–e767 (2013).
Handler, S. M., Kane-Gill, S. L. & Kellum, J. A. Optimal and early detection of acute kidney injury requires effective clinical decision support systems. Nephrol. Dial. Transplant. 29, 1802–1803 (2014).
Pai, A. B. Keeping kidneys safe: the pharmacist's role in NSAID avoidance in high-risk patients. J. Am. Pharm. Assoc. 55, e15–23 (2015).
Jang, S. M. et al. NSAID-avoidance education in community pharmacies for patients at high risk for acute kidney injury, upstate New York, 2011. Prev. Chronic Dis. 11, E220 (2014).
Author information
Authors and Affiliations
Consortia
Contributions
All authors made equal contributions to discussion of content and researching data for the article. L.S.C., R.B., A.B., S.L.G. and E.H. wrote the article and L.S.C., R.B., S.L.G., S.M.B., J.K. and C.R. reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.S.C. has received consulting fees from Astute Medical, Nxstage Medical and Bard Medical. R.B has received research support from Baxter Medical. A.B. has received research support from Astute Medical, INC and the Society of Critical Care Medicine. S.L.G. has received grants from Gambro Renal Products, VitaFlo, Mallinckrodt, and Alexion; grants and personal fees from Baxter Healthcare, Astute Medical, Bellco, AM Pharma, and La Jolla Pharmaceuticals; and personal fees from Bioporto, Akebia, Otsuka, Kaneka, and MediBeacon, all outside the submitted work. S.M.B. has received consulting fees from Baxter and La Jolla Pharmaceuticals. L.F. has received receiving consulting fees from Fresenius, Astute Medical, Eras Pharma, Baxter, and Ortho Clinical. E.H. has received speaker's fees from Alexion and a research grant from Bellco. J.K. has received consulting fees from Nxstage Medical, Astute Medical, Sphingotec and Pfizer. E.M. has received research funding from Relypsa and Fresenius Kabi. R.M. has received consulting fees from Eli Lilly, Spectral, Keryx, Baxter, Ardea, AM Pharma, GSK, CSL Behring, Grifols, Astute Inc. Fresenius-Kabi, Quark, Ferring Research, Ionis Pharmaceuticals, Johnsons and Johnson and DURECT, and has received research support from iSAEC, Relypsa, Fresenius-Kabi and Fresenius. M.O. has received speaker honoraria and research funding from Fresenius Medical Care, and payment for advisory services from Baxter/Gambro. P.M.P has received consulting fees from Durect and Baxter. R.W. has received consulting fees from Durect and MedBeacon and unrestricted research support from Baxter. A.Z. has received unrestricted research grants from Fresenius and Astute Medical and lecture fees from Fresenius, Braun, Astute Medical, and Astellas. C.R. has received consulting fees from Astute, Asahi, GE and Baxter. J.A.K. has received consulting fees from Astute Medical, Atoc Bio, Astellas, Bard Medical, Baxter Medical, Bioporto, Grifols, Medibeacon and Nxstage Medical. E.D.S., D.B., D.C., Z.E., R.L.F., S.L.K.-G., K.D.L., P.M, M.N., N.P. and M.R. declare no competing interests.
Supplementary information
Supplementary information S1 (table)
Supplementary information S1–S6 (tables) (PDF 165 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chawla, L., Bellomo, R., Bihorac, A. et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13, 241–257 (2017). https://doi.org/10.1038/nrneph.2017.2
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.2
This article is cited by
-
Sepsis-associated acute kidney injury: recent advances in enrichment strategies, sub-phenotyping and clinical trials
Critical Care (2024)
-
cGAS-STING, inflammasomes and pyroptosis: an overview of crosstalk mechanism of activation and regulation
Cell Communication and Signaling (2024)
-
Vitamin D metabolism in critically ill patients with acute kidney injury: a prospective observational study
Critical Care (2024)
-
Mortality and mode of dialysis: meta-analysis and systematic review
BMC Nephrology (2024)
-
The intervention of cannabinoid receptor in chronic and acute kidney disease animal models: a systematic review and meta-analysis
Diabetology & Metabolic Syndrome (2024)