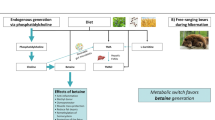
Key Points
-
Biomimetic studies of non-laboratory wild animals are useful for identifying mechanisms that protect or increase susceptibility to disease
-
Domestic and captive felids are vulnerable to chronic kidney disease (CKD), supporting the hypothesis that high protein intake — particularly from red meats and in combination with dehydration — is nephrotoxic
-
Extreme models of ageing, such as Hutchinson–Gilford progeria syndrome and the naked mole rat, can be used to investigate the mechanisms of vascular progeric processes in CKD
-
Current evidence suggests that elevated serum phosphate levels promote ageing and cellular senescence
-
The transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) may offer protection against diseases in extreme environmental conditions and may promote longevity in the animal kingdom; NRF2 agonists (such as resveratrol and sulforaphane) might improve the uraemic complications of CKD
-
Lipid composition of membranes has a role in seasonal acclimatization of metabolic activities in the animal kingdom
-
Hibernating wild bears with anuria are protected against many of the complications observed in humans with CKD, such as muscle wasting, osteoporosis and azotaemia; future studies should investigate the mechanisms behind these protective effects
Abstract
Many of the >2 million animal species that inhabit Earth have developed survival mechanisms that aid in the prevention of obesity, kidney disease, starvation, dehydration and vascular ageing; however, some animals remain susceptible to these complications. Domestic and captive wild felids, for example, show susceptibility to chronic kidney disease (CKD), potentially linked to the high protein intake of these animals. By contrast, naked mole rats are a model of longevity and are protected from extreme environmental conditions through mechanisms that provide resistance to oxidative stress. Biomimetic studies suggest that the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) offers protection in extreme environmental conditions and promotes longevity in the animal kingdom. Similarly, during months of fasting, immobilization and anuria, hibernating bears are protected from muscle wasting, azotaemia, thrombotic complications, organ damage and osteoporosis — features that are often associated with CKD. Improved understanding of the susceptibility and protective mechanisms of these animals and others could provide insights into novel strategies to prevent and treat several human diseases, such as CKD and ageing-associated complications. An integrated collaboration between nephrologists and experts from other fields, such as veterinarians, zoologists, biologists, anthropologists and ecologists, could introduce a novel approach for improving human health and help nephrologists to find novel treatment strategies for CKD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stenvinkel, P. & Johnson, R. J. Kidney biomimicry — a rediscovered scientific field that could provide hope to patients with kidney disease. Arch. Med. Res. 44, 584–590 (2013).
Krogh, A. The progress of physiology. Am. J. Physiol. 90, 243–251 (1929).
Smith, H. W. Comparative physiology of the kidney. JAMA 153, 1512–1514 (1953).
Sperber, I. Studies on the mammalian kidney. Zool. Bidrag Uppsala 22, 249–432 (1944).
O'Connor, T. P., Lee, A., Jarvis, J. U. & Buffenstein, R. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 835–842 (2002).
Davis, R. W., Castellini, M. A., Kooyman, G. L. & Maue, R. Renal glomerular filtration rate and hepatic blood flow during voluntary diving in Weddell seals. Am. J. Physiol. 245, R743–R748 (1983).
Stenvinkel, P., Jani, A. H. & Johnson, R. J. Hibernating bears (Ursidae): metabolic magicians of definite interest for the nephrologist. Kidney Int. 83, 207–212 (2013).
Stenvinkel, P. et al. Metabolic changes in summer active and anuric hibernating free-ranging brown bears (Ursus arctos). PLOS One 8, e72934 (2013).
Levin, A. et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 390, 1888–1917 (2017).
Stenvinkel, P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J. Intern. Med. 268, 456–467 (2010).
Kooman, J. P., Kotanko, P., Schols, A. M., Shiels, P. G. & Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10, 732–742 (2014).
Lew, Q. J. et al. Red meat intake and risk of ESRD. J. Am. Soc. Nephrol. 28, 304–312 (2017).
Goraya, N. & Wesson, D. E. Is dietary red meat kidney toxic? J. Am. Soc. Nephrol. 28, 5–7 (2017).
Reynolds, B. S. & Lefebvre, H. P. Feline CKD: pathophysiology and risk factors — what do we know? J. Feline Med. Surg. 15 (Suppl. 1), 3–14 (2013).
Jiménez, A. et al. Membranous glomerulonephritis in the Iberian lynx (Lynx pardinus). Vet. Immunol. Immunopathol. 121, 34–43 (2008).
Chetboul, V. et al. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J. Vet. Intern. Med. 17, 89–95 (2003).
Cannon, A. B., Westropp, J. L., Ruby, A. L. & Kass, P. H. Evaluation of trends in urolith composition in cats: 5,230 cases J. Am. Vet. Assoc. 231, 570–576 (2007).
Brown, C. A., Elliott, J., Schmiedt, C. W. & Brown, S. A. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet. Pathol. 53, 309–326 (2016).
Munson, L. et al. Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J. Wildl. Dis. 41, 542–548 (2005).
Waki, M. F., Martorelli, C. R., Mosko, P. E., Erdmann, P. & Kogika, M. M. Classification into stages of chronic kidney disease in dogs and cats — clinical, laboratorial and therapeutic approach (Portuguese). Cienia Rural 40, 2226–2234 (2010).
Junginger, J. et al. Pathology in captive wild felids at German zoological gardens. PLOS One 10, e0130573 (2015).
Elliott, J. & Barber, P. J. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J. Small Anim. Pract. 39, 78–85 (1998).
Zini, E. et al. Renal morphology in cats with diabetes mellitus. Vet. Pathol. 51, 1143–1150 (2014).
Bolton, L. A. & Munson, L. Glomerulosclerosis in captive cheetahs (Acinonyx jubatus). Vet. Pathol. 36, 14–22 (1999).
Oaks, J. L. et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633 (2004).
Eisert, R. Hypercarnivory and the brain: protein requirements of cats reconsidered. J. Comp. Physiol. B 181, 1–17 (2011).
Meyer, T. W., Lawrence, W. E. & Brenner, B. M. Dietary protein and the progression of renal disease. Kidney Int. Suppl. 16, S243–S247 (1983).
Kramer, H. Kidney disease and the westernization and industrialization of food. Am. J. Kidney Dis. 70, 111–121 (2017).
DiBartola, S. P., Buffington, C. A., Chew, D. J., McLoughlin, M. A. & Sparks, R. A. Development of chronic renal disease in cats fed a commercial diet. J. Am. Vet. Med. Assoc. 202, 744–751 (1993).
Juraschek, S. P., Appel, L. J., Anderson, C. A. & Miller, E. R. Effect of a high-protein diet on kidney function in healthy adults: results from the OmniHeart trial. Am. J. Kidney Dis. 61, 547–554 (2013).
Brenner, B. M., Meyer, T. W. & Hostetter, T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 307, 652–659 (1982).
Mafra, D. et al. Red meat intake in chronic kidney disease patients: two sides of the coin. Nutrition 46, 26–32 (2018).
Haring, B. et al. Dietary protein sources and risk for incident chronic kidney disease: results from the atherosclerosis risk in communities (ARIC) study. J. Ren Nutr. 4, 233–242 (2017).
Rebholz, C. M. et al. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am. J. Kidney Dis. 68, 853–861 (2016).
Lin, C. K. et al. Comparison of renal function and other health outcomes in vegetarians versus omnivores in Taiwan. J. Health Popul. Nutr. 28, 470–475 (2010).
Kontessis, P. et al. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 38, 136–144 (1990).
Azadbakht, L., Atabak, S. & Esmaillzadeh, A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care 31, 648–654 (2008).
Nongouch, A. & Davenport, A. The effect of vegetarian diet on skin autofluorescence measurements in haemodialysis patients. Br. J. Nutr. 113, 1040–1043 (2015).
Gluba-Brzózka, A., Franczyk, B. & Rysz, J. Vegetarian diet in chronic kidney disease — a friend or foe. Nutrients 9, 374 (2017).
Larsson, S. C. & Orsini, N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am. J. Epidemiol. 179, 282–289 (2014).
Crippa, A., Larsson, S. C., Discacciati, A., Wolk, A. & Orsini, N. Red and processed meat consumption and risk of bladder cancer: a dose-response meta-analysis of epidemiological studies. Eur. J. Nutr. https://doi.org/10.1007/s00394-016-1356-0 (2016).
Kaluza, J., Wolk, A. & Larsson, S. C. Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke 43, 2556–2560 (2012).
Micha, R., Wallace, S. K. & Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 121, 2271–2283 (2010).
Micha, R., Michas, G. & Mozaffarian, D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes — an updated review of the evidence. Curr. Atheroscler Rep. 14, 515–524 (2012).
de Mello, V. D., Zelmanovitz, T., Perassolo, M. S., Azevedo, M. J. & Gross, J. L. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am. J. Clin. Nutr. 83, 1032–1038 (2006).
de Mello, V. D., Zelmanovitz, T., Azevedo, M. J., de Paula, T. P. & Gross, J. L. Long-term effect of a chicken-based diet versus enalapril on albuminuria in type 2 diabetic patients with microalbuminuria. J. Renal Nutr. 18, 440–447 (2008).
Elliott, J., Rawlings, J. M., Markwell, P. J. & Barber, P. J. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J. Small Anim. Pract. 41, 235–242 (2000).
Alisson-Silva, F., Kawanishi, K. & Varki, A. Human risk of diseases associated with red meat intake: analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Mol. Aspects Med. 51, 16–30 (2016).
Tang, W. H. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 116, 448–455 (2015).
Sun, X. et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 481, 63–70 (2016).
Missailidis, C. et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLOS One 11, e0141738 (2016).
Wang, Z. et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163, 1585–1595 (2015).
Koeth, R. A. et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 20, 799–812 (2014).
Martin, O. C. et al. Antibiotic suppression of intestinal microbiota reduces heme-induced lipoperoxidation associated with colon carcinogenesis in rats. Nutr. Cancer 67, 119–125 (2015).
McClelland, R. et al. Accelerated ageing and renal dysfunction links lower socioeconomic status and dietary phosphate intake. Aging 8, 1135–1149 (2016).
Martínez-Moreno, J. M. et al. High phosphate induces a pro-inflammatory response by vascular smooth muscle cells and modulation by vitamin D derivatives. Clin. Sci. 131, 1449–1463 (2017).
Choi, H. K., Atkinson, K., Karlson, E. W., Willett, W., & Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 350, 1093–1103 (2004).
Hammett, F. S. The nitrogen excretion of the cat during a purine-free and a purine-rich diet. J. Biol. Chem. 22, 551–558 (1915).
Villegas, R. et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men's Health Study. Nutr. Metab. Cardiovasc. Dis. 22, 409–416 (2012).
Clifford, A. J., Riumallo, J. A., Youn, V. R. & Scrimshaw, N. S. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J. Nutr. 106, 428–450 (1976).
Nakanishi, N. et al. Low urine pH Is a predictor of chronic kidney disease. Kidney Blood Press Res. 35, 77–81 (2012).
Appel, S. L., Houston, D. M., Moore, A. E. & Weese, J. S. Feline urate urolithiasis. Can. Vet. J. 51, 493–496 (2010).
Osborne, C. A., Lulich, J. P., Ulrich, L. K., & Bird, K. A. Feline crystalluria. Detection and interpretation. Vet. Clin. North Am. Small Anim. Pract. 26, 369–391 (1996).
Ryu, E. S. et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am. J. Physiol. Renal Physiol. 304, F471–F480 (2013).
Greene, J. P. et al. Risk factors associated with the development of chronic kidney disease in cats evaluated at primary care veterinary hospitals. J. Am. Vet. Med. Assoc. 244, 320–327 (2014).
Campese, V. M. Con: Mesoamerican nephropathy: is the problem dehydration or rehydration? Nephrol. Dial Transplant 32, 603–606 (2017).
Johnson, R. J. Heat stress as a potential etiology of Mesoamerican and Sri Lankan nephropathy: a late night consult with Sherlock Holmes. Nephrol. Dial Transplant 32, 598–602 (2017).
McFarland, W. N., Wimsatt, W., A. Urine flow and composition in the vampire bat. Am. Zool. 5, 662–667 (1965).
Singer, M. A. Vampire, bat, shrew, and bear: comparative physiology and chronic renal failure. Am. J. Physiol. Regulatory Integrative Comp. Physiol. 282, R1583–R1592 (2002).
Hoff, C. C. & Reidesel, M. L. (eds) Physiological Systems in Semiarid Environments. (University of New Mexico Press, 1969).
Holloway, B. W. & Ripley, S. H. Nucleic acid content of reticulocytes and its relation to uptake of radioactive leucine in vitro. J. Biol. Chem. 196, 695–701 (1952).
Fulop, T. et al. Aging, frailty and age-related diseases. Biogerontology 11, 547–563 (2010).
Shiels, P. G., McGuiness, D., Eriksson, M., Kooman, J. P. & Stenvinkel, P. The role of epigenetics in renal ageing. Nat. Rev. Nephrol. 13, 471–482 (2017).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell Metab. 153, 1194–1217 (2013).
Sturmlechner, I., Durik, M., Sieben, C. J., Baker, D. J. & van Deursen, J. M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 13, 77–89 (2017).
Stenvinkel, P. & Larsson, T. Chronic kidney disease: a clinical model of premature aging. Am. J. Kidney Dis. 62, 339–351 (2013).
Karam, Z. & Tuazon, J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 29, 555–564 (2013).
Kaplan, C., Pasternack, B., Shah, H. & Gallo, G. Age-related incidence of sclerotic glomeruli in human kidneys. Am. J. Pathol. 80, 227–234 (1975).
Kooman, J. et al. Inflammation and premature aging in advanced chronic kidney disease. Am. J. Physiol. Renal Physiol. 313, F938–F950 (2017).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13, 629–646 (2017).
Glassock, R. J. & Rule, A. D. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 82, 270–277 (2012).
Roncal-Jimenez, C. A. et al. Aging-associated renal disease in mice is fructokinase dependent. Am. J. Physiol. Renal Physiol. 311, F722–F730 (2016).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Sosnowska, D. et al. A heart that beats for 500 years: age-related changes in cardiac proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in Arctica islandica, the longest-living noncolonial animal. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1448–1461 (2014).
Nielsen, J. et al. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science 353, 702–704 (2016).
Finch, C. E. Update on slow aging and negligible senescence — a mini-review. Gerontology 55, 307–313 (2009).
Valenzano, D. R. et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 16, 296–300 (2006).
Buffenstein, R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B 4, 439–445 (2008).
Jarvis, J. U. M. & Bennet, N. C. in The Biology of the Naked Mole-Rat. (eds Sherman, P. W., Jarvis, J. U. M. & Alexander, R. D.) 66–96 (Princeton University Press, 1991).
Yahav, S., Buffenstein, R. & Pettifor, J. M. Calcium and inorganic phosphorus metabolism in naked mole rats Hetercephalus glaber is only indirectly affected by cholecalciferol. Gen. Comp. Endocrinol. 89, 161–166 (1993).
De Waal, E. M. et al. Elevated protein carbonylation and oxidative stress do not affect protein structure and function in the long-living naked-mole rat: a proteomic approach. Biochem. Biophys. Res. Commun. 434, 815–819 (2013).
Triplett, J. C. et al. Age-related changes in the proteostasis network in the brain of the naked mole-rat: implications promoting healthy longevity. Biochim. Biophys. Acta 1852, 2213–2224 (2015).
Dai, D. F., Wessells, R. J., Bodmer, R. & Rabinovitch, P. S. in The Comparative Biology of Aging. (ed Wolf N. S.) 259–286 (Springer, 2010).
Grimes, K. M., Lindsey, M. L., Gelfond, J. A. & Buffenstein, R. Getting to the heart of the matter: age-related changes in diastolic heart function in the longest-lived rodent, the naked mole rat. J. Gerontol. A Biol. Sci. Med. Sci. 67, 384–394 (2012).
Grimes, K. M., Reddy, A. K., Lindsey, M. L. & Buffenstein, R. And the beat goes on: maintained cardiovascular function during aging in the longest-lived rodent, the naked mole-rat. Am. J. Physiol. Heart Circ. Physiol. 307, H284–291 (2014).
Lagunas-Rangel, F. A. & Chávez-Valencia, V. Learning of nature: the curious case of the naked mole rat. Mech. Ageing Dev. 164, 76–81 (2017).
Skulachev, V. P. et al. Neoteny, prolongation of youth: from naked mole rats to “naked apes” (humans). Physiol. Rev. 97, 699–720 (2017).
Comfort, A. The biology of senescence. 3rd edn (Elsevier, 1979).
Tian, X. et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349 (2013).
Itoh, K., Ye, P., Matsumiya, T., Tanji, K., & Ozaki, T. Emerging functional cross-talk between the Keap1–Nrf2 system and mitochondria. J. Clin. Biochem. Nutr. 56, 91–97 (2015).
Lewis, K. N., Mele, J., Hayes, J. D., & Buffenstein, R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr. Comp. Biol. 50, 829–843 (2010).
Lewis, K. N. et al. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc. Natl Acad. Sci. USA 112, 3722–3727 (2015).
Pomatto, L. C. D., Tower, J. & Davies, K. J. A. Sexual dimorphism and aging differentially regulate adaptive homeostasis. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glx083 (2017).
Kubben, N. et al. Repression of the antioxidant Nrf2 pathway in premature aging. Cell 165, 1361–1374 (2016).
Kubben, N. & Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat. Rev. Mol. Cell Biol. 18, 595–609 (2017).
Chopra, A. & Lineweaver, C. H. in Proceedings of the 8th Australian Space Science Conference. 1st edn (eds Short, W. & Cairns, I.) 49–55 (National Space Society of Australia Ltd, 2008).
Ohno, S. The reason for as well as the consequence of the cambrian explosion in animal evolution. J. Mol. Evol. 44, S23–S27 (1997).
Bowen, H. J. M. Environmental Chemistry of the Elements. (Academic Press, 1979).
Benner, S. A., Ellington, A. D. & Tauer, A. Modern metabolism as a palimpsest of the RNA world. Proc. Natl Acad. Sci. USA 86, 7054–7058 (1989).
Blair-West, J. R. et al. Behavioral and tissue responses to severe phosphorus depletion in cattle. Am. J. Physiol. 263, R656–R663 (1992).
Hu, M. C., Shiizaki, K., Kuro-o M. & Moe, O. W. Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 75, 503–533 (2013).
Swapna, M. Applied Mineralogy: Applications in Industry and Environment. (Springer, 2011).
Lenton, S., Nylander, T., Teixeira, S. C. & Holt, C. A review of the biology of calcium phosphate sequestration with special reference to milk. Dairy Sci. Technol. 95, 3–14 (2015).
Kuro-o, M., Matsumura, Y. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Kuro-o, M. The FGF23 and Klotho system beyond mineral metabolism. Clin. Exp. Nephrol. 21, 64–69 (2017).
Maltese, G. et al. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J. Cell. Mol. Med. 21, 621–627 (2017).
Beck, G. R., Moran, E. & Knecht, N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp. Cell Res. 288, 288–300 (2003).
Shanahan, C. M. Mechanisms of vascular calcification in CKD — evidence for premature ageing? Nat. Rev. Nephrol. 9, 661–670 (2013).
Stenvinkel, P. et al. CDKN2A/p16INK4a expression is associated with vascular progeria in chronic kidney disease. Aging 9, 494–507 (2017).
Troyano, N. et al. Hyperphosphatemia induces cellular senescence in human aorta smooth muscle cells through integrin linked kinase (ILK) up-regulation. Mech. Ageing Dev. 152, 43–55 (2015).
Di Marco, G. S. et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Renal Physiol. 294, F1381–F1387 (2008).
Jono, S. et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 87, E10–E17 (2000).
Sage, A. P., Lu, J., Tintut, Y. & Demer, L. L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 79, 414–422 (2011).
Villa-Bellosta, R. & Sorribas, V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler. Thromb. Vasc. Biol. 29, 761–766 (2009).
Yamada, S. et al. Phosphate binders prevent phosphate-induced cellular senescence of vascular smooth muscle cells and vascular calcification in a modified, adenine-based uremic rat model. Calcif. Tissue Int. 96, 347–358 (2015).
Jeyapalan, J. C. & Sedivy, J. M. Cellular senescence and organismal aging. Mech. Ageing Dev. 129, 467–474 (2008).
Kuro-o, M. A potential link between phosphate and aging — lessons from Klotho-deficient mice. Mech. Ageing Dev. 131, 270–275 (2010).
Merideth, M. A. et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N. Engl. J. Med. 358, 592–604 (2008).
Villa-Bellosta, R. et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 127, 2442–2451 (2013).
Chang, A. R., Lazo, M., Appel, L. J., Gutierrez, O. M. & Grams, M. E. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am. J. Clin. Nutr. 99, 320–327 (2014).
Hamano, T. et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J. Am. Soc. Nephrol. 21, 1998–2007 (2010).
Smith, E. R., Ford, M. L., Tomlinson, L. A., Rajkumar, C., McMahon, L. P., & Holt, S. G. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol. Dial. Transplant. 27, 1957–1966 (2012).
Smith, E. R., Hanssen, E., McMahon, L. P. & Holt, S. G. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLOS One 8, e60904 (2013).
Smith, E. R. et al. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 25, 339–348 (2014).
Teare, J. A. ISIS Reference Ranges for Physiological Values in Captive Wildlife (Electronic Resource). (International Species Information System, 2002).
Moe, S. M. et al. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int. Suppl. 113, S1–S130 (2009).
Fouque, D., Horne, R., Cozzolino, M. & Kalantar-Zadeh, K. Balancing nutrition and serum phosphorus in maintenance dialysis. Am. J. Kidney Dis. 64, 143–150 (2014).
Peter, W. L. S., Wazny, L. D., Weinhandl, E., Cardone, K. E. & Hudson, J. Q. A. Review of phosphate binders in chronic kidney fisease: incremental progress or just higher costs? Drugs 14, 329–345 (2016).
Kawai, M., Kinoshita, S., Ozono, K. & Michigami, T. Inorganic phosphate activates the AKT/mTORC1 pathway and shortens the life span of an α-Klotho-deficient model. J. Am. Soc. Nephrol. 27, 2810–2824 (2016).
ter Braake, A. D., Shanahan, C. M. & de Baaij, J. H. F. Magnesium counteracts vascular calcification: passive interference or active modulation? Arterioscler. Thromb. Vasc. Biol. 37, 1431–1445 (2017).
Miller, M. et al. Changes in serum calcium, phosphorus, and magnesium levels in captive ruminants affected by diet manipulation. J. Zoo Wildl. Med. 41, 404–408 (2010).
Koh, G. Y. & Rowling, M. J. Resistant starch as a novel dietary strategy to maintain kidney health in diabetes mellitus. Nutr. Rev. 75, 350–360 (2017).
Bilinski, T., Paszkiewicz, T. & Zadrag-Ecza, R. Energy excess is the main cause of accelerated aging of mammals. Oncotarget 6, 12090–12919 (2016).
Stenvinkel, P., Kooman, J. P. & Shiels, P. G. Nutrients and ageing: what can we learn about ageing interactions from animal biology? Curr. Opin. Clin. Nutr. Metab. Care 19, 19–25 (2016).
Mattison, J. A. et al. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14603 (2017).
Lanaspa, M. A. et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287, 40732–40744 (2012).
Sanchez-Lozada, L. G. et al. Uric Acid-Induced Endothelial Dysfunction Is Associated with Mitochondrial Alterations and Decreased Intracellular ATP Concentrations. Nephron. Exp. Nephrol. 121, e71–e78 (2012).
Johnson, R. J. The Fat Switch. (Mercola.com, 2012).
Dolinsky, V. W. et al. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J. Physiol. 590, 2783–2799 (2012).
Hall, J. A., Dominy, J. E., Lee, Y. & Puigserver, P. The sirtuin family's role in aging and age-associated pathologies. J. Clin. Invest. 123, 973–979 (2013).
Narkar, V. A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Cantó, C. & Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 20, 325–331 (2009).
Kulkarni, S. R., Armstrong, L. E. & Slitt, A. Caloric restriction-mediated induction of lipid metabolism gene expression in liver is enhanced by Keap1-knockdown. Pharm. Res. 30, 2221–2231 (2013).
Sanchez-Roman, I. & Barja, G. Regulation of longevity and oxidative stress by nutritional interventions: role of methionine restriction. Exp. Gerontol. 48, 1030–1042 (2013).
Yang, G. et al. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal 18, 1906–1919 (2013).
Hine, C. et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 (2015).
Pietri, R., Román-Morales, E. & López-Garriga, J. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid. Redox Signal 15, 393–404 (2011).
Wallace, J. L. & Wang, R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 14, 329–345 (2015).
McIsaac, R. S., Lewis, K. N., Gibney, P. A. & Buffenstein, R. From yeast to human: exploring the comparative biology of methionine restriction in extending eukaryotic life span. Ann. NY Acad. Sci. 1363, 155–170 (2016).
Dziegelewska, M. et al. Low sulfide levels and a high degree of cystathionine β-synthase (CBS) activation by S-adenosylmethionine (SAM) in the long-lived naked mole-rat. Redox Biol. 8, 192–198 (2016).
Pamplona, R. & Barja, G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine conenction. Biochim. et Biophys. Acta 1757, 496–508 (2006).
Valli, A. et al. Elevated serum levels of S-adenosylhomocysteine, but not homocysteine, are associated with cardiovascular disease in stage 5 chronic kidney disease patients. Clin. Chim. Acta 395, 106–110 (2008).
Suliman, M. E., Filho, J. C., Bárány, P., Lindholm, B. & Bergström, J. Effects of methionine loading on plasma and erythrocyte sulphur amino acids and sulph-hydryls before and after co-factor supplementation in haemodialysis patients. Nephrol. Dial Transplant 16, 102–110 (2001).
Brown-Borg, H. M. & Buffenstein, R. Cutting back on the essentials: can manipulating intake of specific amino acids modulate health and lifespan? Aging Res. Rev. 39, 87–95 (2017).
Weber, G. J., Pushpakumar, S. B. & Sen, U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am. J. Physiol. Heart Circ. Physiol. 312, H874–H885 (2017).
Cooney, C. A. Are somatic cells inherently deficient in methylation metabolism? A proposed mechanism for DNA methylation loss, senescence and aging. Growth Dev. Aging 57, 261–273 (1993).
Park, T. J. et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356, 307–311 (2017).
Pfeiffer, C. J. Renal cellular and tissue specializations in the bottlenose dolphin (Tursiops truncatus) and the beluga whale (Delphinapteras leucas). Aquatic Mammals 23, 75–84 (1997).
Andrews, M. T., Russeth, K. P., Drewes, L. R. & Henry, P. G. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R383–393 (2009).
Jani, A. et al. Renal protection from prolonged cold ischemia and warm reperfusion in hibernating squirrels. Transplantation 92, 1215–1221 (2011).
Vázquez-Medina, J. P. et al. Prolonged fasting activates Nrf2 in post-weaned elephant seals. J. Exp. Biol. 216, 2870–2878 (2013).
Vázquez-Medina, J. P., Zenteno-Savín, T., Elsner, R. & Ortiz, R. M. Coping with physiological oxidative stress: a review of antioxidant strategies in seals. J. Comp. Physiol. B 182, 741–750 (2012).
Vázquez-Medina, J. P., Zenteno-Savín, T., Forman, H. J., Crocker, D. E. & Ortiz, R. M. Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. J. Exp. Biol. 214, 1294–1299 (2011).
Nezu, M. et al. Transcription factor Nrf2 hyperactivation in early-phase renal ischemia-reperfusion injury prevents tubular damage progression. Kidney Int. 91, 387–401 (2017).
Cirillo, P. et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J. Am. Soc. Nephrol. 20, 545–553 (2009).
Nigro, D. et al. Chronic administration of saturated fats and fructose differently affect SREBP activity resulting in different modulation of Nrf2 and Nlrp3 inflammasome pathways in mice liver. J. Nutr. Biochem. 42, 160–171 (2017).
Perez-Pinzon, M. A. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147, 291–299 (2007).
Geiser, F. & Ruf, T. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol. Zool. 68, 935–966 (1995).
Turbill, C., Ruf, T., Mang, T. & Arnold, W. Regulation of heart rate and rumen temperature in red deer: effects of season and food intake. J. Exp. Biol. 214, 963–970 (2011).
Signer, C., Ruf, T. & Arnold, W. Hypometabolism and basking: the strategies of alpine ibex to endure harsh over-wintering conditions. Funct. Ecol. 25, 537–547 (2011).
Arnold, W. et al. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R174–R181 (2004).
Arnold, W. in Life in the Cold: Ecological, Physiological, and Molecular Mechanisms. (eds Carey, C., Florant, G. L., Wunder, B. A. & Horwitz, B.) 65–80. (Westview Press, 1993).
Parker, K. L., Barboza, P. S. & Gillingham, M. P. Nutrition integrates environmental responses of ungulates. Funct. Ecol. 23, 57–69 (2009).
Arnold, W. et al. Contrary seasonal changes of rates of nutrient uptake, organ mass, and voluntary food intake in red deer (Cervus elaphus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R277–R285 (2015).
Loudon, A. S. I. Photoperiod and the regulation of annual and circannual cycles of food intake. Proc. Nutr. Soc. 53, 495–507 (1994).
Hume, D. et al. Seasonal changes in morphology and function of the gastrointestinal tract of free-living alpine marmots (Marmota marmota). J. Comp. Physiol. B 172, 197–207 (2002).
Rigano, K. S. et al. Life in the fat lane: seasonal regulation of insulin sensitivity, food intake, and adipose biology in brown bears. J. Comp. Physiol. B 187, 649–676 (2017).
Sommer, F. et al. The gut microbiota modulates energy metabolism in the hibernating brown bear Ursus arctos. Cell Rep. 14, 1655–1661 (2016).
Toien, O. et al. Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331, 906–909 (2011).
Arnold, W., Ruf, T., Frey-Roos, F. & Bruns, U. Diet-independent remodeling of cellular membranes precedes seasonally changing body temperature in a hibernator. PLOS One 6, e18641 (2011).
Arnold, W., Giroud, S., Valencak, T. G. & Ruf, T. Ecophysiology of omega fatty acids: a lid for every jar. Physiology 30, 232–240 (2015).
Helge, J. W. et al. Training affects muscle phospholipid fatty acid composition in humans. J. Appl. Physiol. 90, 670–677 (2001).
Mitchell, T. W., Buffenstein, R. & Hulbert, A. J. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): a comparative study using shotgun lipidomics. Exp. Gerontol. 42, 1053–1062 (2007).
Giroud, S. et al. Membrane phospholipid fatty acid composition regulates cardiac SERCA activity in a hibernator, the Syrian hamster (Mesocricetus auratus). PLOS One 8, e63111 (2013).
Arnold, W., Ruf, T. & Kuntz, R. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) II. Energy expenditure. J. Exp. Biol. 209, 4566–4573 (2006).
Maillet, D. & Weber, J. M. Relationship between n-3 PUFA content and energy metabolism in the flight muscles of a migrating shorebird: evidence for natural doping. J. Exp. Biol. 210, 413–420 (2007).
Hulbert, A. J., Kelly, M. A. & Abbott, S. K. Polyunsaturated fats, membrane lipids and animal longevity. J. Comp. Physiol. B 184, 149–166 (2014).
Chen, D. Q. et al. The link between phenotype and fatty acid metabolism in advanced chronic kidney disease. Nephrol. Dial Transplant. 32, 1154–1166 (2017).
Gao, L. et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 282, 2529–2537 (2007).
Andersen, J. B., Rourke, B. C., Caiozzo, V. J., Bennett, A. F. & Hicks, J. W. Postprandial cardiac hypertrophy in pythons. Nature 434, 37 (2005).
Riquelme, C. A. et al. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science 334, 528–531 (2011).
Hall, J. C. & Rosbash, M. Oscillating molecules and how they move corcadian clocks across evolutionary boundaries. Proc. Natl Acad. Sci. USA 90, 5382–5383 (1993).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 (2005).
Pekovic-Vaughan, V. et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 28, 548–560 (2014).
Gumz, M. L. Molecular basis of circadian rhythmicity in renal physiology and pathophysiology. Exp. Physiol. 101, 1025–1029 (2016).
Smolensky, M. H., Hermida, R. C., Reinberg, A., Sackett-Lundeen, L. & Portaluppi, F. Circadian disruption: new clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol. Int. 33, 1101–1119 (2016).
Obi, Y. et al. Seasonal variations in transition, mortality and kidney transplantation among patients with end-stage renal disease in the USA. Nephrol. Dial. Transplant. 32, 907 (2017).
Evans, A. L. et al. Drivers of hibernation in the brown bear. Front. Zool. 13, 7 (2016).
Arinell, K. et al. Brown bears (Ursus arctos) seem resistant to atherosclerosis despite highly elevated plasma lipids during hibernation and active state. Clin. Transl Sci. 5, 269–272 (2012).
Iles, T. L., Laske, T. G., Garschelis, D. L. & Iaizzo, P. A. Blood clotting behavior is innately modulated in Ursus americanus during early and late denning relative to summer months. J. Exp. Biol. 220, 455–459 (2017).
Brown, D. C., Mulhausen, R. O., Andrew, D. J. & Seal, U. S. Renal function in anesthetized dormant and active bears. Am. J. Physiol. 220, 293–298 (1971).
Prunescu, C., Serban-Parau, N., Brock, J. H., Vaughan, D. M. & Prunescu, P. Liver and kidney structure and iron content in romanian brown bears (Ursus arctos) before and after hibernation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 134, 21–26 (2003).
Ortiz, R. M. Osmoregulation in marine mammals. J. Exp. Biol. 204, 1831–1844 (2001).
Dugbartey, G. J. et al. Renal mitochondrial response to low temperature in non-hibernating and hibernating species. Antioxid. Redox Signal 27, 599–617 (2017).
Walford, R. L. & Spindler, S. R. The response to caloric restriction in mammals shows features also common to hibernation: a cross-adaption hypothesis. J. Gerontol. A Biol. Sci. Med. Sci. 52, B179–B183 (1997).
Turbilli, C., Bieber, C. & Ruf, T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. 278, 3355–3363 (2011).
Storey, K. B. & Storey, J. M. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Quarterly Rev. Biol. 65, 145–174 (1990).
Blackstone, E., Morrison, M. & Roth, M. B. H2S induces a suspended animation-like state in mice. Science 308, 518 (2005).
Blackstone, E. & Roth, M. B. Suspended animation-like state protects mice from lethal hypoxia. Shock 27, 370–372 (2007).
Shimada, S. et al. Hydrogen sulfide augments survival signals in warm ischemia and reperfusion of the mouse liver. Surg. Today 45, 892–903 (2015).
Xu, R. et al. Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Exp. Neurol. 247, 392–401 (2013).
Dugbartey, G. J., Bouma, H. R., Strijkstra, A. M., Boerema, A. S. & Henning, R. H. Induction of a torpor-like state by 5′-AMP does not depend on H2S production. PLOS One 21, e01366113 (2015).
Huang, L. et al. The AMPK agonist PT1 and mTOR Inhibitor 3HOI-BA-01 protect cardiomyocytes after ischemia through induction of autophagy. J. Cardiovasc. Pharmacol. Ther. 21, 70–81 (2016).
Ratigan, E. D. & McKay, D. B. Exploring principles of hibernation for organ preservation. Transpl. Rewiews 30, 13–19 (2016).
Harlow, H. J., Lohuis, T., Beck, T. D. I. & Iaizzo, P. A. Muscle strength in overwintering bears. Nature 409, 997 (2001).
Nelson, R., Wahner, H. W., Jones, J. D., Ellefson, R. D. & Zollman, P. E. Metabolism of bears before, during, and after winter sleep. Am. J. Physiol. 224, 491–496 (1973).
Lin, D. C., Hershey, J. D., Mattoon, J. S. & Robbins, C. T. Skeletal muscles of hibernating brown bears are unusually resistant to effects of denervation. J. Exp. Biol. 215, 2081–2087 (2012).
Fuster, G., Busquets, S., Almendro, V., Lopez-Soriano, F. J. & Argilés, J.M. Antiproteolytic effects of plasma from hibernating bears: a new approach for muscle wasting therapy? Clin. Nutr. 26, 658–661 (2007).
Andres-Mateos, E. et al. Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol. Med. 5, 80–91 (2013).
Ivakine, E. A. & Cohn, R. D. Maintaining skeletal muscle mass: lessons learned from hibernation. Exp. Physiol. 99, 632–637 (2014).
Luo, J. et al. Serum glucocorticoid-regulated kinase 1 blocks CKD-Induced muscle wasting via inactivation of FoxO3a and Smad2/3. J. Am. Soc. Nephrol. 27, 2797–2808 (2016).
Chung, N., Park, J. & Lim, K. The effects of exercise and cold exposure on mitochondrial biogenesis in skeletal muscle and white adipose tissue. J. Exerc. Nutr. Biochem. 21, 39–47 (2017).
Tran, M. T. et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531, 528–532 (2016).
Gidlund, E. K. et al. Rapidly elevated levels of PGC-1α-b protein in human skeletal muscle after exercise: exploring regulatory factors in a randomized controlled trial. J. Appl. Physiol. 119, 374–384 (2015).
Oh, S. et al. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 7, 12902 (2017).
McGee Lawrence, M. E. et al. Six months of disuse during hibernation does not increase intracortical porosity or decrease cortical bone geometry, strength or mineralization in black bears (Ursus americanus) femurs. J. Biomech. 42, 1378–1383 (2009).
McGee-Lawrence, M. et al. Suppressed bone remodeling in black bears conserves energy and bone mass during hibernation. J. Exp. Biol. 218, 2067–2074 (2015).
Fedorov, V. B. et al. Preservation of bone mass and structure in hibernating black bears (Ursus americanus) through elevated expression of analoic genes. Funct. Integr. Genom. 12, 357–365 (2012).
Seger, R. et al. Investigating the mechanisms for maintaing eucalcemia despite immobility and anuria in the hibernating American black bear (Ursus americanus). Bone 49, 1205–1212 (2011).
Donahue, S. W. et al. Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J. Exp. Biol. 209, 1630–1638 (2006).
Gray, S. K. et al. Black bear parathyroid hormone has greater anabolic effects on trabecular bone in dystrophin-deficient mice than in wild type mice. Bone 51, 578–585 (2012).
Ibánez, L. et al. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid. Med. Cell. Longev. 2014, 726590 (2014).
Thummuri, D., Naidu, V. G. M. & Chaudhari, P. Carnosic acid attenuates RANKL-induced oxidative stress and osteoclastogenesis via induction of Nrf2 and suppression of NF-κB and MAPK signalling. J. Mol. Med. 95, 1065–1076 (2017).
Ni, Z. & Storey, K. B. Heme oxygenase expression and Nrf2 signaling during hibernation in ground squirrels. Can. J. Physiol. Pharmacol. 88, 379–387 (2010).
Iaizzo, P. A., Laske, T. G., Harlow, H. J., McClay, C. B. & Garshelis, D. L. Wound healing during hibernation by black bears (Ursus americanus) in the wild: elicitation of reduced scar formation. Integr. Zool. 7, 48–60 (2012).
Barboza, P. S., Farley, S. D. & Robbins, C. T. Whole-body urea cycling and protein turnover during hyperphagia and dormancy in growing bears (Ursus americanus and U. arctos). Canadian J. Zoology 75, 2129–2136 (1997).
Nelson, R. A., Jones, J. D., Wahner, H. W., McGill, D. B. & Code, C. F. Nitrogen metabolism in bears: urea metabolism in summer starvation and in winter sleep and role of urinary bladder in water and nitrogen conservation. Mayo Clin. Proc. 50, 141–146 (1975).
Spector, D. A., Deng, J., Coleman, R. & Wade, J. B. The urothelium of a hibernator: the American black bear. Physiol. Rep. 3, e12429 (2015).
Walser, M. Urea metabolism in chronic renal failure. J. Am. Soc. Nephrol. 9, 1544–1551 (1998).
Ahlquist, D. A., Nelson, R. A., Steiger, D. L., Jones, J. D. & Ellefson, R. D. Glycerol metabolism in the hibernating black bear. J. Comp. Physiol. 155, 75–79 (1984).
Nelson, R. A., Beck, T. D. I. & Steiger, D. L. Ratio of serum urea to serum creatinine in wild black bears. Science 226, 841–842 (1984).
Nakagawa, T., Lomb, D. J., Haigis, M. C. & Guarente, L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 (2009).
Van Tighem, K. Food and Diet. The Get Bear Smart Society http://www.bearsmart.com/about-bears/food-diet/.
Carlson, S. M. Synchronous timing of food resources triggers bears to switch from salmon to berries. Proc. Natl Acad. Sci. USA 114, 10309–10311 (2017).
Lattanzio, V., Lattanzio, V. M. T. & Cardinali, A. in Phytochemistry: Advances in Research. (ed Imperato, F.) 23–67 (Research Signpost, 2006).
Overall, J. et al. Metabolic effects of berries with structurally diverse anthocyanins. Int. J. Mol. Sci. 15, E422 (2017).
Durbin, S. M. et al. Resveratrol supplementation preserves long bone mass, microstructure, and strength in hindlimb-suspended old male rats. J. Bone Miner. Metab. 32, 38–47 (2014).
Lee, S. G. et al. Relationship between oxidative stress and bone mass in obesity and effects of berry supplementation on bone remodeling in obese male mice: an exploratory study. J. Med. Food 18, 476–482 (2015).
Moriwaki, S. et al. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLOS One 13, e97177 (2014).
Murata, M. et al. Delphinidin prevents muscle atrophy and upregulates miR-23a expression. J. Agr. Food Chem. 65, 45–50 (2017).
Alvarado, J. L. et al. Delphinidin-rich maqui berry extract (Delphinol®) lowers fasting and postprandial glycemia and insulinemia in prediabetic individuals during oral glucose tolerance Tests. Biomed. Res. Int. 2016, 9070537 (2016).
Ali, B. H. et al. Effect of aqueous extract and anthocyanins of calyces of Hibiscus sabdariffa (Malvaceae) in rats with adenine-induced chronic kidney disease. J. Pharm. Pharmacol. 69, 1219–1229 (2017).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Zhu, Y. et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–659 (2015).
Chang, X. Y. et al. Quercetin attenuates vascular calcification through suppressed oxidative stress in adenine-induced chronic renal failure rats. Biomed. Res. Int. 2017, 5716204 (2017).
Momken, I. et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 10, 3646–3660 (2011).
Cheng, K. H. et al. Resveratrol ameliorates metabolic disordes and muscle wasting in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 301, E853–E863 (2011).
Sen, C. K., Khanna, S., Gordillo, G., Bagchi, D., Bagchi, M. & Roy, S. Oxygen, oxidants, and antioxidants in wound healing: an emerging paradigm. Ann. NY Acad. Sci. 957, 239–249 (2002).
Basu, A. et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 140, 1582–1587 (2010).
Erlund, I. et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 87, 323–331 (2008).
Stull, A. J., Cash, K. C., Johnson, W. D., Champagne, C. M. & Cefalu, W. T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 140, 1764–1768 (2010).
Cassidy, A. et al. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 127, 188–196 (2013).
Wang, X., Rybczynski, N., Harington, C.R., White, S.C. & Tedford, R.H. A basal ursine bear (Protarctos abstrusus) from the pliocene high arctic reveals eurasian affinities and a diet rich in fermentable sugars. Sci. Rep. 7, 17722 (2017).
Reiter, R. J. et al. Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Rev. Nutr. Diet 97, 211–230 (2007).
Pedruzzi, L. M. et al. Systemic inflammation and oxidative stress in hemodialysis patients are associated with down-regulation of Nrf2. J. Nephrol. 28, 495–501 (2015).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant of uremia: oxidative stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 62, 1524–1538 (2002).
Noel, S., Hamad, A. R. & Rabb, H. Reviving the promise of transcription factor Nrf2-based therapeutics for kidney diseases. Kidney Int. 88, 1217–1218 (2015).
Axelsson, A. S. et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 9, eeah4477 (2017).
Sun, W. et al. Pomegranate extract decreases oxidative stress and alleviates mitochondrial impairment by activating AMPK-Nrf2 in hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Sci. Rep. 6, 34246 (2016).
Ali, B. H. et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Bas. Clin. Pharmacol. Toxicol. 122, 65–73 (2018).
Han, C. W. et al. Ethanol extract of Alismatis Rhizoma reduces acute lung inflammation by suppressing NF-κB and activating Nrf2. J. Ethnopharmacol 146, 402–410 (2013).
Wondrak, G. T. et al. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules 15, 3338–3355 (2010).
Esgalhado, M., Stenvinkel, P. & Mafra, D. Nonpharmacologic strategies to modulate nuclear factor eryhroid 2-related factor 2 pathway in chronic kidney disease. J. Ren. Nutr. 27, 282–291 (2017).
Kwon, J. S. et al. Sulforaphane inhibits restenosis by suppressing inflammation and the proliferation of vascular smooth muscle cells. Atherosclerosis 225, 41–49 (2012).
Juurlink, B. H. Dietary Nrf2 activators inhibit atherogenic processes. Atherosclerosis 225, 29–33 (2012).
Pomatto, L. C. D. et al. The age- and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging 9, 1153–1185 (2017).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013).
O'Mealey, G. B. et al. PGAM5-KEAP1-Nrf2 complex is required for stress-induced mitochondrial retrograde trafficking. J. Cell Sci. 130, 3467–3480 (2017).
Vaziri, N. D. et al. Dose-dependent deleterious and salutary actions of the Nrf2 inducer dh404 in chronic kidney disease. Free Radic. Biol. Med. 86, 374–381 (2015).
Tebay, L. E. et al. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 88, 108–146 (2015).
Acknowledgements
The authors thank the Scandinavian Brown Bear Project (in particular, O. Fröbert, J. E. Swenson, S. Brunberg, J. M. Arnemo and A. Zedrosser). P.S.'s research benefits from support from the Swedish Medical Research Council, the Heart and Lung Foundation, Njurfonden and EU-funded INTRICARE projects. R.J.J. and M.L. benefit from research support from the Veterans Administration (BX002586), Department of Defense (PR130106), US National Institutes of Health (NIH) (DK108859 and DK109408), La Isla Foundation, Solidaridad and the Danone Research Foundation. M.K. is supported by the Japan Agency for Medical Research and Development (AMED) Core Research for Evolutionary Medical Science and Technology (CREST), AMED, and the Japan Society for the Promotion of Science (16H05302, 16K15470). W.A.'s research has benefited from the grant 'Polyunsaturated fatty acids and seasonal acclimatization' (30061-B25).
Author information
Authors and Affiliations
Contributions
P.S. and R.J.J. launched the idea of studying renal biomimetics. P.S., J.P., M.K., M.L., W.A., T.R., P.G.S. and R.J.J. researched the literature, discussed the content of the article and wrote the text. All authors reviewed or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
P. S. received grants and honoraria from Baxter, Bayer, Astra Zeneca, Bristol-Myers Squibb, Pfizer, Akeiba and Corvidia. M.K. has received grants and honoraria from Bayer, Astellas, Bristol-Myers Squibb and Kissei Pharmaceuticals. R.J.J. has grants from the US National Institutes of Health (NIH), Department of Defense and the Veteran's Administration. He is also a member of Colorado Research Partners, LLC, which is developing inhibitors of fructose metabolism. The other authors declare no competing interests.
Supplementary information
Supplementary information tables
Supplementary information S1–S5 (table) (PDF 409 kb)
Glossary
- Uraemic phenotype
-
Phenotype that includes several physical characteristics, such as vascular stiffness, sarcopenia, frailty, osteoporosis and left ventricular hypertrophy.
- Chronic tubulointerstitial fibrosis
-
Diseases that affect the physiology of non-glomerular structures (tubules and/or the interstitium) in the kidney.
- Glomerular haemodynamics
-
The regulation of efferent and afferent glomerular arteriolar resistance required to maintain a stable glomerular filtration rate.
- Urinary specific gravity
-
Test that compares the density of urine to that of water.
- N-Nitroso compounds
-
Compounds found in processed meat that are formed endogenously from the intake of nitrite and nitrate.
- Nutrigenomic compounds
-
Bioactive nutrients that have an effect on or interact with the genome. Nutrigenomics also encompasses the effect of genetic variations on the absorption, metabolism, elimination or biological effects of various nutrients.
- Telomere attrition
-
Telomeres are the protective endcaps of chromosomes. Attrition, or shortening, of telomeres is a form of tumour suppression and may be due to inflammation and oxidative stress as well as exposure to infectious agents, resulting in limited stem cell function, regeneration and organ maintenance during ageing.
- Uraemic milieu
-
Toxic internal milieu in patients with uraemia that is characterized by accumulation of uraemic toxins and waste products that promote inflammation, oxidative stress, carbonylation, calcification and endothelial dysfunction.
- Senescent cells
-
Cellular senescence is an irreversible cell cycle arrest mechanism that acts to protect against cancer. Senescent cells also have a role in complex biological processes, such as development, tissue repair and age-related disorders.
- Hypercapnia
-
Abnormally elevated carbon dioxide (CO2) levels in the blood.
- High-molecular-weight hyaluronan
-
A high-molecular-weight polysaccharide found in the extracellular matrix, especially in soft connective tissues.
- Antagonistic pleiotropy
-
Scenarios in which one gene contributes to multiple traits, whereby at least one of these traits is beneficial and at least one is detrimental to the organism's health.
- Phosphate appetite
-
A well-documented behaviour in animals that is induced by phosphate deficiency, which is especially common among herbivores.
- Protein–energy wasting
-
A process characterized by a decline in body protein mass and energy reserves, including muscle and fat wasting and loss of visceral proteins. Protein energy wasting is often associated with inflammation and is a strong predictor of mortality.
- Caloric restriction
-
A reduction in calorie intake without incurring malnutrition or a reduction in essential nutrients. In a variety of species, such yeast, fish, rodents and dogs, calorie restriction has been shown to slow the biological ageing process.
- Sirtuin
-
Sirtuins (or NAD+-dependent histone deacetylases) are a class of proteins that possess deacylase activity and regulate important biological pathways and cellular processes, including ageing, inflammation, transcription and apoptosis. Sirtuin agonists include pterostilbene and resveratrol.
- Trans-sulfuration pathway
-
A metabolic pathway that involves the interconversion of homocysteine and cysteine via the intermediate cystathionine.
- S-Sulfhydration
-
A post-translational modification that increases the catalytic activity of proteins. Physiological actions of sulfhydration include the regulation of endoplasmic reticulum stress signalling, inflammation and vascular tension.
- One-carbon methyl donor units
-
DNA methylation influences the expression of some genes and depends upon the availability of methyl groups. Dietary methyl groups are derived from food sources that contain methionine, one-carbon units, choline or betaine (a choline metabolite).
- Torpor
-
A state of reduced body temperature and metabolic rate in animals that enables them to survive periods of reduced food availability.
- Circadian clock
-
The circadian clock regulates the internal and external activities of organisms, such as sleep and changes in metabolism, based on the day–night cycle.
- Chronotherapy
-
The science of timing drugs according to the circadian clock. This approach is used in various clinical conditions, such as cancer, hypertension, seasonal affective disorder and bipolar disorder.
- Renal lobulation
-
Carnivores and most small mammals have smooth-surfaced and uni-pyramidal kidneys, whereas primates and Suidae (hogs and pigs) have a smooth-surfaced and multi-pyramidal kidney system. Large terrestrial mammals have multi-lobulated and multi-pyramidal kidneys to keep the proximal convoluted tubules short. Most marine mammals and bears have each lobe separated into renules (reniculated kidney system).
- Therapeutic hypothermia
-
(also known as targeted temperature management). The induction of mild hypothermia (32–35 °C) after cardiac arrest for neuroprotection.
- Sedentary behaviour
-
A type of behaviour that is characterized by an energy expenditure ≤1.5 metabolic equivalents while in a lying, reclining or sitting posture. Typical sedentary behaviours include watching TV, computer work, driving and reading.
- Denervation
-
Loss of nerve supply to a part of the body, which can be due to multiple causes, such as surgery, physical injury, chemical toxicity or diseases.
- Disuse atrophy
-
A type of muscle atrophy that occurs when a muscle is less active than usual. Disuse atrophy is a common feature in chronic debilitating diseases and immobility.
- Mechanical unloading
-
A mechanical manoeuvre or therapy that decreases tissue growth and regeneration. Whereas mechanical loading of mammalian tissues is a potent promoter of tissue growth and regeneration, mechanical unloading in microgravity causes reduced tissue regeneration via stem cell tissue progenitors.
- Eucalcaemia
-
The maintenance of normal and constant serum calcium levels.
- Blueberries
-
Blueberries comprise all blue-coloured berries of the Vaccinium genus, of which the most common is bilberries. Blueberries have a low glycaemic index and are a rich source of fibres, vitamin K, manganese, >15 different anthocyanins (especially delphinidin and malvidin), quercetin, myricetin and resveratrol.
- Anthocyanins
-
Anthocyanins (>600 molecular structures) belong to a class of molecules called flavonoids that are universal plant colourants responsible for the red, purple and blue colours in many fruits, berries, vegetables and flowers. Due to their contribution in multiple physiological activities, the consumption of these molecules is believed to have a substantial role in preventing lifestyle-related diseases.
- Senolytic effects
-
Senolytic compounds selectively induce the death of senescent cells.
Rights and permissions
About this article
Cite this article
Stenvinkel, P., Painer, J., Kuro-o, M. et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol 14, 265–284 (2018). https://doi.org/10.1038/nrneph.2017.169
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.169
This article is cited by
-
Formerly bile-farmed bears as a model of accelerated ageing
Scientific Reports (2023)
-
On-chip construction of a fully structured scaffold-free vascularized renal tubule
Biomedical Microdevices (2023)
-
Transcription factor NRF2 as potential therapeutic target for preventing muscle wasting in aging chronic kidney disease patients
Journal of Nephrology (2022)
-
Loss of αklotho causes reduced motor ability and short lifespan in zebrafish
Scientific Reports (2021)
-
Socioeconomic position links circulatory microbiota differences with biological age
Scientific Reports (2021)