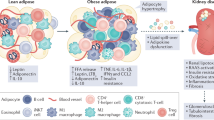
Key Points
-
Adipocytes are metabolically active cells; they produce signalling lipids and metabolites and secrete protein factors (adipokines)
-
The relative levels of these lipids, proteins and metabolites change under different nutritional and pathological states, with adipocytes integrating information regarding the metabolic status quo at any given time and adjusting their cellular physiological state to maintain systemic homeostasis
-
Adipocyte-derived factors establish complex paracrine and endocrine signalling axes between local cell types in adipose tissue and other organs, including the kidney
-
A number of adipokines, including leptin and adiponectin, have well-established effects on kidney function; adiponectin might also be produced locally within the kidney and exert important metabolic functions in an autocrine fashion
-
As a source of pro-inflammatory cytokines, adipose tissue might exert important effects on the inflammatory state of the kidney
-
The renin–angiotensin system is present in adipose tissue and mediates inflammation in response to nutritional interventions; activation of this axis triggers profound signalling events in the kidney
Abstract
Excess adiposity can induce adverse sequelae in multiple cell types and organ systems. The transition from the lean to the obese state is characterized by fundamental cellular changes at the level of the adipocyte. These changes affect the local microenvironment within the respective adipose tissue but can also affect nonadipose systems. Adipocytes within fat pads respond to chronic nutrient excess through hyperplasia or hypertrophy, which can differentially affect interorgan crosstalk between various adipose depots and other organs. This crosstalk is dependent on the unique ability of the adipocyte to coordinate metabolic adjustments throughout the body and to integrate responses to maintain metabolic homeostasis. These actions occur through the release of free fatty acids and metabolites during times of energy need — a process that is altered in the obese state. In addition, adipocytes release a wide array of signalling molecules, such as sphingolipids, as well as inflammatory and hormonal factors (adipokines) that are critical for interorgan crosstalk. The interactions of adipose tissue with the kidney — referred to as the adipo–renal axis — are important for normal kidney function as well as the response of the kidney to injury. Here, we discuss the mechanistic basis of this interorgan crosstalk, which clearly has great therapeutic potential given the increasing rates of chronic kidney disease secondary to obesity and type 2 diabetes mellitus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Hall, M. E. et al. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renovasc. Dis. 7, 75–88 (2014).
Maric-Bilkan, C. Obesity and diabetic kidney disease. Med. Clin. North Am. 97, 59–74 (2013).
Wickman, C. & Kramer, H. Obesity and kidney disease: potential mechanisms. Semin. Nephrol. 33, 14–22 (2013).
Satirapoj, B. et al. Obesity and its relation to chronic kidney disease: a population-based, cross-sectional study of a Thai army population and relatives. Nephrology (Carlton) 18, 229–234 (2013).
Wang, Y., Chen, X., Song, Y., Caballero, B. & Cheskin, L. J. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 73, 19–33 (2008). The findings of this meta-analysis suggest overweight and obesity are associated with a high risk of kidney disease.
World Health Organization. Obesity and overweight — fact sheet. WHO http://www.who.int/mediacentre/factsheets/fs311/en (2017).
D'Agati, V. D. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 12, 453–471 (2016).
Tran, M.-H., Foster, C. E., Kalantar-Zadeh, K. & Ichii, H. Kidney transplantation in obese patients. World J. Transplant. 6, 135–143 (2016).
Chagnac, A. et al. The effects of weight loss on renal function in patients with severe obesity. J. Am. Soc. Nephrol. 14, 1480–1486 (2003).
Tirosh, A. et al. Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes Care 36, 2225–2232 (2013).
Morales, E. & Praga, M. The effect of weight loss in obesity and chronic kidney disease. Curr. Hypertens. Rep. 14, 170–176 (2012).
Teta, D. Weight loss in obese patients with chronic kidney disease: who and how? J. Ren. Care 36 (Suppl. 1), 163–171 (2010).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Cohen, P. & Spiegelman, B. M. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes 64, 2346–2351 (2015).
Giordano, A., Smorlesi, A., Frontini, A., Barbatelli, G. & Cinti, S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 170, R159–R171 (2014).
Hausman, D. B., DiGirolamo, M., Bartness, T. J., Hausman, G. J. & Martin, R. J. The biology of white adipocyte proliferation. Obes. Rev. 2, 239–254 (2001).
Cinti, S. The adipose organ. Prostaglandins Leukot. Essent. Fatty Acids 73, 9–15 (2005).
Ussar, S. et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl Med. 6, 247ra103 (2014).
de Jong, J. M. A., Larsson, O., Cannon, B. & Nedergaard, J. A stringent validation of mouse adipose tissue identity markers. Am. J. Physiol. Endocrinol. Metab. 308, E1085–E1105 (2015).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Dadson, P. et al. Effect of bariatric surgery on adipose tissue glucose metabolism in different depots in patients with or without type 2 diabetes. Diabetes Care 39, 292–299 (2016).
O'Rourke, R. W. et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-γ in inflammation in human adipose tissue. Int. J. Obes. (Lond.). 33, 978–990 (2009).
Wijayatunga, N. N. et al. Adipose depot-specific differences in transcriptome and microRNA expression in high fat diet induced obese mice. FASEB J. 30, 622–626 (2016).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
Jeffery, E. et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 24, 142–150 (2016).
Ferrante, A. W. Jr. The immune cells in adipose tissue. Diabetes. Obes. Metab. 15 (Suppl. 3), 34–38 (2013).
Huh, J. Y., Park, Y. J., Ham, M. & Kim, J. B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells 37, 365–371 (2014).
Cipolletta, D. Adipose tissue-resident regulatory T cells: phenotypic specialization, functions and therapeutic potential. Immunology 142, 517–525 (2014).
Becker, M., Levings, M. K. & Daniel, C. Adipose-tissue regulatory T cells: critical players in adipose-immune crosstalk. Eur. J. Immunol. http:dx.doi.org/10.1002/eji.201646739 (2017).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Murano, I. et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 49, 1562–1568 (2008).
Amano, S. U. et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 (2014).
Oh, D. Y., Morinaga, H., Talukdar, S., Bae, E. J. & Olefsky, J. M. Increased macrophage migration into adipose tissue in obese mice. Diabetes 61, 346–354 (2012).
Zheng, C. et al. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 7, e2167 (2016).
Zamarron, B. F. et al. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes 66, 392–406 (2017).
Magkos, F. et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 23, 591–601 (2016).
Bradley, D. et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J. Clin. Invest. 122, 4667–4674 (2012).
Haase, J. et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia 57, 562–571 (2014).
Tardelli, M. et al. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Mol. Metab. 5, 1131–1137 (2016).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissuemacrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Lesna, I. K. et al. Human adipose tissue accumulation is associated with pro-inflammatory changes in subcutaneous rather than visceral adipose tissue. Nutr. Diabetes 7, e264 (2017).
Kralova Lesna, I. et al. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J. Transl Med. 14, 208 (2016).
Shankar, A., Syamala, S., Xiao, J. & Muntner, P. Relationship between plasma leptin level and chronic kidney disease. Int. J. Nephrol. 2012, 269532 (2012).
Bluher, M. Adipokines — removing road blocks to obesity and diabetes therapy. Mol. Metab. 3, 230–240 (2014).
Fasshauer, M. & Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 36, 461–470 (2015).
Wang, G.-X., Zhao, X.-Y. & Lin, J. D. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol. Metab. 26, 231–237 (2015).
Villarroya, F., Cereijo, R., Villarroya, J. & Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13, 26–35 (2017).
Pajvani, U. B. et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11, 797–803 (2005). This study uses inducible fatless mice to study the roles of adipocytes in the regulation of multiple pathophysiological functions.
Wernstedt Asterholm, I. et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–118 (2014). This paper establishes the important beneficial roles of inflammation on adipose tissues.
Virtue, S. et al. Prostaglandin profiling reveals a role for haematopoietic prostaglandin D synthase in adipose tissue macrophage polarisation in mice and humans. Int. J. Obes. (Lond.) 39, 1151–1160 (2015).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 (2011).
Nasrallah, R., Hassouneh, R. & Hebert, R. L. Chronic kidney disease: targeting prostaglandin E2 receptors. Am. J. Physiol. Renal Physiol. 307, F243–F250 (2014).
Mothe-Satney, I. et al. Adipocytes secrete leukotrienes: contribution to obesity-associated inflammation and insulin resistance in mice. Diabetes 61, 2311–2319 (2012).
Sharma, J. N. & Mohammed, L. A. The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology 14, 10–16 (2006).
Ying, W. et al. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J. Clin. Invest. 127, 1019–1030 (2017).
Mehmood, Z. H. & Papandreou, D. An updated mini review of vitamin D and obesity: adipogenesis and inflammation state. Open Access Maced. J. Med. Sci. 4, 526–532 (2016).
Abbas, M. A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 165, 369–381 (2017).
Mittendorfer, B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr. Opin. Clin. Nutr. Metab. Care 14, 535–541 (2011).
Stern, J. H., Rutkowski, J. M. & Scherer, P. E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 23, 770–784 (2016). This is a timely overview of the importance of adipocyte-derived factors.
Erion, D. M. & Shulman, G. I. Diacylglycerol-mediated insulin resistance. Nat. Med. 16, 400–402 (2010).
Bikman, B. T. & Summers, S. A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Invest. 121, 4222–4230 (2011).
Mitsnefes, M. et al. Ceramides and cardiac function in children with chronic kidney disease. Pediatr. Nephrol. 29, 415–422 (2014).
Afshinnia, F. et al. Lipidomic signature of progression of chronic kidney disease in the chronic renal insufficiency cohort. Kidney Int. Rep. 1, 256–268 (2016).
Deng, Y. et al. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 355, eaaf5375 (2017).
Kimura, T. et al. Identification of biomarkers for development of end-stage kidney disease in chronic kidney disease by metabolomic profiling. Sci. Rep. 6, 26138 (2016).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Sun, K., Tordjman, J., Clement, K. & Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 (2013). This is an important overview summarizing the pathophysiological consequences of adipose tissue fibrosis.
Spencer, M. et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 96, E1990–E1998 (2011).
Chun, T.-H. et al. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125, 577–591 (2006).
Lee, J.-T. et al. Macrophage metalloelastase (MMP12) regulates adipose tissue expansion, insulin sensitivity, and expression of inducible nitric oxide synthase. Endocrinology 155, 3409–3420 (2014).
Niu, H. et al. Matrix metalloproteinase 12 modulates high-fat-diet induced glomerular fibrogenesis and inflammation in a mouse model of obesity. Sci. Rep. 6, 20171 (2016).
Borgeson, E. et al. Lipoxin A4 attenuates adipose inflammation. FASEB J. 26, 4287–4294 (2012).
Borgeson, E. et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab. 22, 125–137 (2015).
Ambarkar, M. et al. Adipokines and their relation to endothelial dysfunction in patients with chronic kidney disease. J. Clin. Diagn. Res. 10, BC04-8 (2016).
Zhao, H.-L. et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 74, 467–477 (2008). This study demonstrates fat redistribution and beiging phenotypes in the progression of renal injury, indicating the adiporenal crosstalk and therapeutic interventions that prevent fat redistribution might ameliorate chronic renal dysfunction.
Kir, S. et al. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab. 23, 315–323 (2016).
Xiang, D. M. et al. Chronic kidney disease promotes chronic inflammation in visceral white adipose tissue. Am. J. Physiol. Renal Physiol. 312, F689–F701 (2017).
D'Apolito, M. et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Invest. 120, 203–213 (2010).
Engeli, S., Negrel, R. & Sharma, A. M. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension 35, 1270–1277 (2000).
Yvan-Charvet, L. & Quignard-Boulange, A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 79, 162–168 (2011).
Yiannikouris, F. et al. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R244–R251 (2012).
Massiera, F. et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 15, 2727–2729 (2001).
Kihara, M. et al. Genetic deficiency of angiotensinogen produces an impaired urine concentrating ability in mice. Kidney Int. 53, 548–555 (1998).
Cole, B. K. et al. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension 55, 715–721 (2010).
Guo, H. et al. Protective effects of glucagon-like peptide-1 analog on renal tubular injury in mice on high-fat diet. Cell. Physiol. Biochem. 41, 1113–1124 (2017).
Ma, L.-J. et al. Angiotensin type 1 receptor modulates macrophage polarization and renal injury in obesity. Am. J. Physiol. Renal Physiol. 300, F1203–F1213 (2011).
Azushima, K. et al. Adipocyte-specific enhancement of angiotensin II type 1 receptor-associated protein ameliorates diet-induced visceral obesity and insulin resistance. J. Am. Heart Assoc. 6, e004488 (2017).
Maeda, A. et al. Angiotensin receptor-binding protein ATRAP/Agtrap inhibits metabolic dysfunction with visceral obesity. J. Am. Heart Assoc. 2, e000312 (2013).
Uneda, K. et al. Angiotensin II type 1 receptor-associated protein regulates kidney aging and lifespan independent of angiotensin. J. Am. Heart Assoc. 6, e006120 (2017).
Kobayashi, R. et al. An angiotensin II type 1 receptor binding molecule has a critical role in hypertension in a chronic kidney disease model. Kidney Int. 91, 1115–1125 (2017).
AbdAlla, S., Lother, H., Abdel-tawab, A. M. & Quitterer, U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 276, 39721–39726 (2001).
Benndorf, R. A. et al. Angiotensin II type 2 receptor deficiency aggravates renal injury and reduces survival in chronic kidney disease in mice. Kidney Int. 75, 1039–1049 (2009).
Ali, Q., Dhande, I., Samuel, P. & Hussain, T. Angiotensin type 2 receptor null mice express reduced levels of renal angiotensin II type 2 receptor/angiotensin (1–7)/mas receptor and exhibit greater high-fat diet-induced kidney injury. J. Renin Angiotensin Aldosterone Syst. http:dx.doi.org/10.1177/1470320316661871 (2016).
Yvan-Charvet, L. et al. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology 150, 1421–1428 (2009).
Li, H. et al. Telmisartan ameliorates nephropathy in metabolic syndrome by reducing leptin release from perirenal adipose tissue. Hypertension 68, 478–490 (2016).
Patel, S. N., Ali, Q. & Hussain, T. Angiotensin II type 2-receptor agonist C21 reduces proteinuria and oxidative stress in kidney of high-salt-fed obese zucker rats. Hypertension 67, 906–915 (2016).
Pandey, A. et al. H2AK119 monoubiquitination regulates angiotensin II receptor mediated macrophage infiltration and renal fibrosis in type 2 diabetic rats. Biochimie 131, 68–76 (2016).
Reddy, M. A. et al. Losartan reverses permissive epigenetic changes in renal glomeruli of diabetic db/db mice. Kidney Int. 85, 362–373 (2014).
Wolf, G., Chen, S., Han, D. C. & Ziyadeh, F. N. Leptin and renal disease. Am. J. Kidney Dis. 39, 1–11 (2002).
Serradeil-Le Gal, C. et al. Characterization and localization of leptin receptors in the rat kidney. FEBS Lett. 404, 185–191 (1997).
Hudkins, K. L. et al. BTBR ob/ob mutant mice model progressive diabetic nephropathy. J. Am. Soc. Nephrol. 21, 1533–1542 (2010).
Chua, S. J. et al. A susceptibility gene for kidney disease in an obese mouse model of type II diabetes maps to chromosome 8. Kidney Int. 78, 453–462 (2010).
Lim, C. C. et al. Elevated serum leptin, adiponectin and leptin to adiponectin ratio is associated with chronic kidney disease in Asian adults. PLoS ONE 10, e0122009 (2015).
Menon, V. et al. Factors associated with serum leptin in patients with chronic kidney disease. Clin. Nephrol. 61, 163–169 (2004).
Cumin, F., Baum, H. P. & Levens, N. Leptin is cleared from the circulation primarily by the kidney. Int. J. Obes. Relat. Metab. Disord. 20, 1120–1126 (1996).
Sharma, K. et al. Plasma leptin is partly cleared by the kidney and is elevated in hemodialysis patients. Kidney Int. 51, 1980–1985 (1997).
Pedone, C. et al. Longitudinal association between serum leptin concentration and glomerular filtration rate in humans. PLoS ONE 10, e0117828 (2015).
Cui, H., Lopez, M. & Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 13, 338–351 (2017).
Alhasson, F. et al. 90 — High circulatory leptin mediated NOX-2 promotes kidney inflammation in nonalcoholic fatty liver disease via MiR21-dependent mesangial cell activation. Free Radical Biol. Med. 100, S51 (2016).
Wolf, G. et al. Leptin stimulates proliferation and TGF-β expression in renal glomerular endothelial cells: potential role in glomerulosclerosis. Kidney Int. 56, 860–872 (1999).
Ding, N. et al. Leptin promotes endothelial dysfunction in chronic kidney disease through AKT/GSK3β and β-catenin signals. Biochem. Biophys. Res. Commun. 480, 544–551 (2016).
Kriz, W., Kaissling, B. & Le Hir, M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 121, 468–474 (2011).
Guerrot, D. et al. Progression of renal fibrosis: the underestimated role of endothelial alterations. Fibrogenesis Tissue Repair 5, S15 (2012).
Mou, X. et al. Serum TGF-β1 as a biomarker for type 2 diabetic nephropathy: a meta-analysis of randomized controlled trials. PLoS ONE 11, e0149513 (2016).
Lan, H. Y. & Chung, A. C.-K. TGF-β/Smad signaling in kidney disease. Semin. Nephrol. 32, 236–243 (2012).
Cui, W., Maimaitiyiming, H., Qi, X., Norman, H. & Wang, S. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am. J. Physiol. Renal Physiol. 305, F871–F880 (2013).
Kumpers, P. et al. Leptin is a coactivator of TGF-β in unilateral ureteral obstructive kidney disease. Am. J. Physiol. Renal Physiol. 293, F1355–F1362 (2007).
Briffa, J. F. et al. Acute leptin exposure reduces megalin expression and upregulates TGFβ1 in cultured renal proximal tubule cells. Mol. Cell. Endocrinol. 401, 25–34 (2015).
Chen, K.-H. et al. The AMPK agonist AICAR inhibits TGF-β1 induced activation of kidney myofibroblasts. PLoS ONE 9, e106554 (2014).
Lim, A. K. H. et al. Role of MKK3-p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia 52, 347–358 (2009).
Blanca, A. J. et al. Leptin induces oxidative stress through activation of NADPH oxidase in renal tubular cells: antioxidant effect of L-carnitine. J. Cell. Biochem. 117, 2281–2288 (2016).
Paz-Filho, G., Mastronardi, C. A. & Licinio, J. Leptin treatment: facts and expectations. Metabolism 64, 146–156 (2015).
Liu, J., Lee, J., Salazar Hernandez, M. A., Mazitschek, R. & Ozcan, U. Treatment of obesity with celastrol. Cell 161, 999–1011 (2015).
Lee, J. et al. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 22, 1023–1032 (2016).
Crunkhorn, S. Metabolic disease: leptin sensitizer reverses obesity. Nat. Rev. Drug Discov. 15, 601 (2016).
Greenhill, C. Obesity: new leptin sensitizer identified. Nat. Rev. Endocrinol. 12, 558 (2016).
Cheung, W. W. et al. A pegylated leptin antagonist ameliorates CKD-associated cachexia in mice. J. Am. Soc. Nephrol. 25, 119–128 (2014). This study reveals that blocking leptin signalling by leptin antagonists would benefit CKD.
Lourenco, E. V., Liu, A., Matarese, G. & La Cava, A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc. Natl Acad. Sci. USA 113, 10637–10642 (2016).
Simonds, S. E. et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416 (2014).
Scholze, A., Rattensperger, D., Zidek, W. & Tepel, M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 15, 1617–1622 (2007).
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Bouskila, M., Pajvani, U. B. & Scherer, P. E. Adiponectin: a relevant player in PPARγ-agonist-mediated improvements in hepatic insulin sensitivity? Int. J. Obes. (Lond.) 29, S17–S23 (2005).
Hyun, Y. Y. et al. Serum adiponectin and protein-energy wasting in predialysis chronic kidney disease. Nutrition 33, 254–260 (2016).
Rovin, B. H. et al. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 68, 1825–1833 (2005).
Zoccali, C. et al. Adiponectin is markedly increased in patients with nephrotic syndrome and is related to metabolic risk factors. Kidney Int. Suppl. 63, S98–S102 (2003).
Zoccali, C. et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J. Am. Soc. Nephrol. 13, 134–141 (2002).
Georgoulidou, A. et al. Adiponectin plasma levels and albuminuria in patients with type 2 diabetes and different stages of diabetic kidney disease. J. Nephrol. Ther. 7, 2–7 (2017).
Kim, H. Y. et al. Association of serum adiponectin level with albuminuria in chronic kidney disease patients. Clin. Exp. Nephrol. 20, 443–449 (2016).
Menon, V. et al. Adiponectin and mortality in patients with chronic kidney disease. J. Am. Soc. Nephrol. 17, 2599–2606 (2006).
Sharma, K. et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 118, 1645–1656 (2008).
Halberg, N. et al. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes 58, 1961–1970 (2009).
von Eynatten, M. et al. Urinary adiponectin excretion: a novel marker for vascular damage in type 2 diabetes. Diabetes 58, 2093–2099 (2009).
Panduru, N. M. et al. Urinary adiponectin is an independent predictor of progression to end-stage renal disease in patients with type 1 diabetes and diabetic nephropathy. Diabetes Care 38, 883–890 (2015).
Perri, A. et al. Adiponectin is expressed and secreted by renal tubular epithelial cells. J. Nephrol. 26, 1049–1054 (2013).
Perri, A. et al. Adiponectin secreted by tubular renal cells during LPS exposure worsens the cellular inflammatory damage. J. Nephrol. 29, 185–194 (2016).
Rutkowski, J. M. et al. Adiponectin alters renal calcium and phosphate excretion through regulation of klotho expression. Kidney Int. 91, 324–337 (2016).
Cammisotto, P. G., Londono, I., Gingras, D. & Bendayan, M. Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am. J. Physiol. Renal Physiol. 294, F881–F889 (2008).
Park, H. S. et al. Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J. Transl Med. 14, 176 (2016).
Sopic´, M. et al. Downregulation of AdipoR1 is associated with increased circulating adiponectin levels in serbian chronic kidney disease patients. J. Med. Biochem. 35, 436–442 (2016).
Shen, Y. Y., Charlesworth, J. A., Kelly, J. J., Loi, K. W. & Peake, P. W. Up-regulation of adiponectin, its isoforms and receptors in end-stage kidney disease. Nephrol. Dial. Transplant. 22, 171–178 (2007).
Martinez Cantarin, M. P., Keith, S. W., Waldman, S. A. & Falkner, B. Adiponectin receptor and adiponectin signaling in human tissue among patients with end-stage renal disease. Nephrol. Dial. Transplant. 29, 2268–2277 (2014).
Yamada-Obara, N. et al. Maternal exposure to high-fat and high-fructose diet evokes hypoadiponectinemia and kidney injury in rat offspring. Clin. Exp. Nephrol. 20, 853–861 (2016).
Ohashi, K. et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler. Thromb. Vasc. Biol. 27, 1910–1917 (2007).
Rutkowski, J. M. et al. Adiponectin promotes functional recovery after podocyte ablation. J. Am. Soc. Nephrol. 24, 268–282 (2013).
Nakamaki, S. et al. Adiponectin reduces proteinuria in streptozotocin-induced diabetic Wistar rats. Exp. Biol. Med. (Maywood) 236, 614–620 (2011).
Guo, X. et al. Adiponectin retards the progression of diabetic nephropathy in db/db mice by counteracting angiotensin II. Physiol. Rep. 2, e00230 (2014).
Bijland, S., Mancini, S. J. & Salt, I. P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. (Lond.) 124, 491–507 (2013).
Smith, B. K. & Steinberg, G. R. AMP-activated protein kinase, fatty acid metabolism, and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 20, 248–253 (2017).
Zhu, Q. et al. Adipocyte-deletion of Ip6k1 reduces diet-induced obesity by enhancing AMPK-mediated thermogenesis. J. Clin. Invest. 126, 4273–4288 (2016).
Gauthier, M.-S. et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 404, 382–387 (2011).
Dugan, L. L. et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 123, 4888–4899 (2013).
Decleves, A.-E., Mathew, A. V., Cunard, R. & Sharma, K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J. Am. Soc. Nephrol. 22, 1846–1855 (2011).
Decleves, A.-E. et al. Regulation of lipid accumulation by AMP-activated kinase in high fat diet-induced kidney injury. Kidney Int. 85, 611–623 (2014).
Wu, U. et al. Protective effects of berberine on high fat-induced kidney damage by increasing serum adiponectin and promoting insulin sensitivity. Int. J. Clin. Exp. Pathol. 8, 14486–14492 (2015).
Cameron, K. O. et al. Discovery and preclinical characterization of 6-chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic acid (PF-06409577), a direct activator of adenosine monophosphate-activated protein kinase (AMPK), for the potential treatment of diabetic nephropathy. J. Med. Chem. 59, 8068–8081 (2016).
Salatto, C. T. et al. Selective activation of AMPK β1-containing isoforms improves kidney function in a rat model of diabetic nephropathy. J. Pharmacol. Exp. Ther. 361, 303–311 (2017).
Sas, K. M. et al. Targeted lipidomic and transcriptomic analysis identifies dysregulated renal ceramide metabolism in a mouse model of diabetic kidney disease. J. Proteomics Bioinform. http:dx.doi.org/10.4172/jpb.S14-002 (2015).
Holland, W. L. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2011).
Holland, W. L. et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 6, 267–275 (2017).
Vasiliauskaite-Brooks, I. et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120–123 (2017).
Holland, W. L. & Scherer, P. E. Structural biology: receptors grease the metabolic wheels. Nature 544, 42–44 (2017).
Park, J. et al. Obesity paradox in end-stage kidney disease patients. Prog. Cardiovasc. Dis. 56, 415–425 (2014).
Kalantar-Zadeh, K. et al. The obesity paradox in kidney disease: how to reconcile it with obesity management. Kidney Int. Rep. 2, 271–281 (2017).
Kim, J.-Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Cooper, R. et al. ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. J. Hum. Hypertens. 11, 107–111 (1997).
Ernst, M. C. & Sinal, C. J. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 21, 660–667 (2010).
Zylla, S. et al. Serum chemerin levels are inversely associated with renal function in a general population. Clin. Endocrinol. (Oxf.) http://dx.doi.org/10.1111/cen.13449 (2017).
Ebert, T. et al. Circulating adipocyte fatty acid binding protein is increased in chronic and acute renal dysfunction. Nutr. Metab. Cardiovasc. Dis. 24, 1027–1034 (2014).
Furuhashi, M. et al. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS ONE 6, e27356 (2011).
Furuhashi, M., Saitoh, S., Shimamoto, K. & Miura, T. Fatty acid-binding protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin. Med. Insights Cardiol. 8, 23–33 (2014).
Hashizume, M. & Mihara, M. Atherogenic effects of TNF-α and IL-6 via up-regulation of scavenger receptors. Cytokine 58, 424–430 (2012).
Su, H., Lei, C.-T. & Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front. Immunol. 8, 405 (2017).
Lee, B. T. et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 16, 77 (2015).
Lin, Z. et al. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS ONE 6, e18398 (2011).
Crasto, C., Semba, R. D., Sun, K. & Ferrucci, L. Serum fibroblast growth factor 21 is associated with renal function and chronic kidney disease in community-dwelling adults. J. Am. Geriatr. Soc. 60, 792–793 (2012).
Boucher, J. et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771 (2005).
Bertrand, C., Valet, P. & Castan-Laurell, I. Apelin and energy metabolism. Front. Physiol. 6, 115 (2015).
Chandrasekaran, B., Dar, O. & McDonagh, T. The role of apelin in cardiovascular function and heart failure. Eur. J. Heart Fail. 10, 725–732 (2008).
Lacquaniti, A. et al. Apelin and copeptin as biomarkers of kidney disease. Springer Nature https://link.springer.com/content/pdf/10.1007%2F978-94-007-7699-9_43.pdf (2015).
Szczepanska, M. et al. Evaluation of adipocytokines in children with chronic kidney disease. Endokrynol. Pol. 66, 100–107 (2015).
Axelsson, J. et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 69, 596–604 (2006).
Azuma, K. et al. Correlation between serum resistin level and adiposity in obese individuals. Obes. Res. 11, 997–1001 (2003).
Feng, R. et al. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: a meta-analysis. Diabetes Res. Clin. Pract. 106, 88–94 (2014).
Sengul, E., Duygulu, G., Dindar, S. & Bunul, F. Serum omentin-1, inflammation and carotid atherosclerosis in patients with non-diabetic chronic kidney disease. Ren. Fail. 35, 1089–1093 (2013).
Qasem, A., Farage, S., Elmesallamy, F. A. & Elsaid, H. H. Association of plasma omentin-1 level with insulin resistance in chronic kidney disease patients. Egypt. J. Obes. Diabetes Endocrinol. 1, 72–76 (2015).
Alcelik, A. et al. Serum levels of omentin in end-stage renal disease patients. Kidney Blood Press. Res. 35, 511–516 (2012).
Catoi, A. F. et al. Increased chemerin and decreased omentin-1 levels in morbidly obese patients are correlated with insulin resistance, oxidative stress and chronic inflammation. Clujul Med. 87, 19–26 (2014).
Watanabe, T., Watanabe-Kominato, K., Takahashi, Y., Kojima, M. & Watanabe, R. Adipose tissue-derived omentin-1 function and regulation. Compr. Physiol. 7, 765–781 (2017).
Frey, S. K. et al. Isoforms of retinol binding protein 4 (RBP4) are increased in chronic diseases of the kidney but not of the liver. Lipids Health Dis. 7, 29 (2008).
Graham, T. E. et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354, 2552–2563 (2006).
Chang, Y.-H., Chang, D.-M., Lin, K.-C., Shin, S.-J. & Lee, Y.-J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab. Res. Rev. 27, 515–527 (2011).
Mahmood, N., Junejo, A. M., Jamal, Q. & Awan, R. Association of visfatin with chronic kidney disease in a cohort of patients with and without diabetes. J. Pak. Med. Assoc. 60, 922–926 (2010).
Adeghate, E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr. Med. Chem. 15, 1851–1862 (2008).
Yan, Q.-W. et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56, 2533–2540 (2007).
Bolignano, D. et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 4, 337–344 (2009).
Hasegawa, M. et al. Plasma neutrophil gelatinase-associated lipocalin as a predictor of cardiovascular events in patients with chronic kidney disease. Biomed. Res. Int. 2016, 8761475 (2016).
Saldanha, J. F. et al. The newly identified anorexigenic adipokine nesfatin-1 in hemodialysis patients: are there associations with food intake, body composition and inflammation? Regul. Pept. 173, 82–85 (2012).
Richter, J. et al. Serum levels of the adipokine progranulin depend on renal function. Diabetes Care 36, 410–414 (2013).
Korolczuk, A. & Beltowski, J. Progranulin, a new adipokine at the crossroads of metabolic syndrome, diabetes, dyslipidemia and hypertension. Curr. Pharm. Des. 23, 1533–1539 (2017).
Gomez-Ambrosi, J. et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 92, 3719–3727 (2007).
Kahles, F., Findeisen, H. M. & Bruemmer, D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 3, 384–393 (2014).
Barreto, D. V. et al. Prognostic implication of plasma osteopontin levels in patients with chronic kidney disease. Nephron Clin. Pract. 117, c363–c372 (2011).
Lorenzen, J. et al. Circulating levels of osteopontin are closely related to glomerular filtration rate and cardiovascular risk markers in patients with chronic kidney disease. Eur. J. Clin. Invest. 40, 294–300 (2010).
Nomura, I., Kato, J., Tokashiki, M. & Kitamura, K. Increased plasma levels of the mature and intermediate forms of adrenomedullin in obesity. Regul. Pept. 158, 127–131 (2009).
Dieplinger, B. et al. Pro-A-type natriuretic peptide and pro-adrenomedullin predict progression of chronic kidney disease: the MMKD Study. Kidney Int. 75, 408–414 (2009).
Acknowledgements
The authors are supported by US National Institutes of Health (NIH) Grants R01-DK086629, R01-DK55758, P01-DK088761 and P01-AG051459 as well as by an unrestricted grant from the NovoNordisk Foundation.
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, discussed its content and contributed to the writing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Beiging
-
A biological process that often occurs under cold stress or β-adrenergic stimulation to increase heat production, during which white adipose tissue switches from a unilocular to a multilocular phenotype (with more beige adipocytes).
- M2-like macrophages
-
So-called alternatively activated macrophages that secrete cytokines that decrease inflammation.
- M1-like macrophages
-
M1-polarized macrophages that release cytokines that encourage inflammation.
- Unilocular
-
White adipocytes that display unilocular features, with a single large lipid droplet in the cytoplasm.
- Multilocular
-
Brown and beige adipocytes that display a multilocular phenotype, with many small lipid droplets in the cytoplasm.
- Dominant isoform
-
The major isoform with the most abundant expression within a protein family.
- Acetylation
-
A type of post-translational histone modification that facilitates or inhibits the binding of a protein complex to its histone-binding site via remodelling of the chromatin structure, resulting in altered gene expression.
- Monoubiquitylation
-
A type of post-translational histone modification; some histones, such as histone 2A, can be monoubiquitylated by adding a ubiquitin unit, thus regulating gene transcription by acting on the chromatin structure.
- Leptin resistance
-
A condition that is often found in obesity and diabetes, in which leptin loses its anorectic functions in the brain despite high circulating levels of the hormone.
- Uraemic toxin
-
Uraemic toxins are a group of uraemic retention solutes found in uraemic syndrome that are otherwise excreted by healthy kidneys.
- Berberine
-
A compound found in herbs that increases adiponectin levels and activates 5′-AMP-activated protein kinase catalytic subunit α-1 (PRKAA1; also known as AMPK) and has anti-inflammatory and antidiabetic properties.
- Sphingolipids
-
A class of lipids that can be part of cellular membranes. Moreover, sphingolipids and their metabolites, such as ceramides, sphingosine and sphingosine-1-phosphate, are signalling molecules and are involved in multiple diseases, including obesity, diabetes, cardiovascular disease, cancer and kidney disease.
Rights and permissions
About this article
Cite this article
Zhu, Q., Scherer, P. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat Rev Nephrol 14, 105–120 (2018). https://doi.org/10.1038/nrneph.2017.157
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.157
This article is cited by
-
Inter-organ crosstalk during development and progression of type 2 diabetes mellitus
Nature Reviews Endocrinology (2024)
-
Interorgan communication networks in the kidney–lung axis
Nature Reviews Nephrology (2024)
-
A novel clinical diagnostic marker predicting the relationship between visceral adiposity and renal function evaluated by estimated glomerular filtration rate (eGFR) in the Chinese physical examination population
Lipids in Health and Disease (2023)
-
Interference of a mammalian circRNA regulates lipid metabolism reprogramming by targeting miR-24-3p/Igf2/PI3K-AKT-mTOR and Igf2bp2/Ucp1 axis
Cellular and Molecular Life Sciences (2023)
-
Sexual dimorphism of leptin and adiposity in children between 0 and 10 years: a systematic review and meta-analysis
Biology of Sex Differences (2022)