Key Points
-
Technological advances and the clinical experience with eculizumab have led to a new confidence in therapeutic strategies that target the complement system
-
Several candidate drugs aimed at a wide range of targets and indications have shown promising efficacy in preclinical studies and early clinical trials, and are now moving into late-stage clinical development
-
The number of potential indications for complement therapeutics, including kidney disorders, is growing owing to new genetic and molecular insights as well as clinical data
-
Given the pathological heterogeneity between and even within indications for complement-specific therapies, careful patient stratification will be essential to pave the way towards new therapeutic options
-
The field of complement drug discovery has already overcome several hurdles, and the growing clinical experience will help to assess the remaining and emerging challenges
Abstract
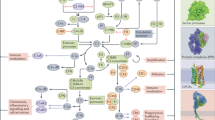
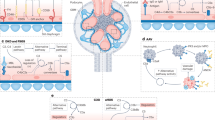
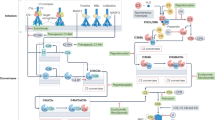
The increasing number of clinical conditions that involve a pathological contribution from the complement system — many of which affect the kidneys — has spurred a regained interest in therapeutic options to modulate this host defence pathway. Molecular insight, technological advances, and the first decade of clinical experience with the complement-specific drug eculizumab, have contributed to a growing confidence in therapeutic complement inhibition. More than 20 candidate drugs that target various stages of the complement cascade are currently being evaluated in clinical trials, and additional agents are in preclinical development. Such diversity is clearly needed in view of the complex and distinct involvement of complement in a wide range of clinical conditions, including rare kidney disorders, transplant rejection and haemodialysis-induced inflammation. The existing drugs cannot be applied to all complement-driven diseases, and each indication has to be assessed individually. Alongside considerations concerning optimal points of intervention and economic factors, patient stratification will become essential to identify the best complement-specific therapy for each individual patient. This Review provides an overview of the therapeutic concepts, targets and candidate drugs, summarizes insights from clinical trials, and reflects on existing challenges for the development of complement therapeutics for kidney diseases and beyond.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ricklin, D. & Lambris, J. D. Complement-targeted therapeutics. Nat. Biotechnol. 25, 1265–1275 (2007).
Rother, R. P., Rollins, S. A., Mojcik, C. F., Brodsky, R. A. & Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 25, 1256–1264 (2007).
Center for Drug Evaluation and Research. Application number: 125166, approval letter. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/125166s0000_APPROV.pdf (2007).
Center for Drug Evaluation and Research. Application number: 125166s172, approval letter. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/bla/2011/125166Orig1s172-2.pdf (2011).
Fakhouri, F. & Fremeaux-Bacchi, V. Thrombotic microangiopathy: eculizumab for atypical haemolytic uraemic syndrome: what next? Nat. Rev. Nephrol. 9, 495–496 (2013).
Reis, E. S., Mastellos, D. C., Ricklin, D., Mantovani, A. & Lambris, J. D. Complement in cancer: untangling an intricate relationship. Nat. Rev. Immunol. http://dx.doi.org/10.1038/nri.2017.97 (2017).
Ricklin, D., Reis, E. S. & Lambris, J. D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12, 383–401 (2016). This Review provides detailed insight into the mechanisms that drive the involvement of complement in various disease processes, including in renal disorders.
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Ricklin, D. & Lambris, J. D. New milestones ahead in complement-targeted therapy. Semin. Immunol. 28, 208–222 (2016).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2015).
Ricklin, D. & Lambris, J. D. Preformed mediators of defense — gatekeepers enter the spotlight. Immunol. Rev. 274, 5–8 (2016).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part I. — molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015).
Blatt, A. Z., Pathan, S. & Ferreira, V. P. Properdin: a tightly regulated critical inflammatory modulator. Immunol. Rev. 274, 172–190 (2016).
Ekdahl, K. N. et al. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol. Rev. 274, 245–269 (2016).
Ricklin, D., Reis, E. S., Mastellos, D. C., Gros, P. & Lambris, J. D. Complement component C3 — the “Swiss army knife” of innate immunity and host defense. Immunol. Rev. 274, 33–58 (2016).
Wang, H., Ricklin, D. & Lambris, J. D. Complement-activation fragment C4a mediates effector functions by binding as untethered agonist to protease-activated receptor 1 and 4. Proc. Natl Acad. Sci. USA 114, 10948–10953 (2017).
Roumenina, L. T., Rayes, J., Frimat, M. & Fremeaux-Bacchi, V. Endothelial cells: source, barrier, and target of defensive mediators. Immunol. Rev. 274, 307–329 (2016). This review describes the intricate relationship between humoral host defence systems, such as complement and coagulation, and endothelial cells, with a particular focus on aHUS and other diseases.
Merle, N. S., Noe, R., Halbwachs-Mecarelli, L., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part II: role in immunity. Front. Immunol. 6, 257 (2015).
Martin, M. & Blom, A. M. Complement in removal of the dead — balancing inflammation. Immunol. Rev. 274, 218–232 (2016).
Scott, D. & Botto, M. The paradoxical roles of C1q and C3 in autoimmunity. Immunobiology 221, 719–725 (2016).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016). This seminal paper on synaptic pruning by the complement system provides an excellent example of the immunoediting role of complement in physiological and pathophysiological situations.
Stephan, A. H., Barres, B. A. & Stevens, B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 35, 369–389 (2012).
Lambris, J. D., Dobson, N. J. & Ross, G. D. Release of endogenous C3b inactivator from lymphocytes in response to triggering membrane receptors for beta 1H globulin. J. Exp. Med. 152, 1625–1644 (1980).
Sundsmo, J. S. Expression of complement on human peripheral blood lymphocytes. Fed. Proc. 39, 1200 (1980). This paper marks an important early milestone in the recognition of complement as a system with intracellular relevance.
Sundsmo, J. S. The leukocyte complement system. Fed. Proc. 41, 3094–3098 (1982).
Freeley, S., Kemper, C. & Le Friec, G. The “ins and outs” of complement-driven immune responses. Immunol. Rev. 274, 16–32 (2016).
Schmidt, C. Q., Lambris, J. D. & Ricklin, D. Protection of host cells by complement regulators. Immunol. Rev. 274, 152–171 (2016).
Ekdahl, K. N., Soveri, I., Hilborn, J., Fellstrom, B. & Nilsson, B. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat. Rev. Nephrol. 13, 285–296 (2017).
Sacks, S. H. & Zhou, W. The role of complement in the early immune response to transplantation. Nat. Rev. Immunol. 12, 431–442 (2012).
Ekdahl, K. N. et al. Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 63, 1042–1050 (2011).
Harris, C. L., Heurich, M., Rodriguez de Cordoba, S. & Morgan, B. P. The complotype: dictating risk for inflammation and infection. Trends Immunol. 33, 513–521 (2012).
Brennan, F. H., Lee, J. D., Ruitenberg, M. J. & Woodruff, T. M. Therapeutic targeting of complement to modify disease course and improve outcomes in neurological conditions. Semin. Immunol. 28, 292–308 (2016).
Patzelt, J., Verschoor, A. & Langer, H. F. Platelets and the complement cascade in atherosclerosis. Front. Physiol. 6, 49 (2015).
Thurman, J. M. Complement in kidney disease: core curriculum 2015. Am. J. Kidney Dis. 65, 156–168 (2015).
Fakhouri, F., Zuber, J., Fremeaux-Bacchi, V. & Loirat, C. Haemolytic uraemic syndrome. Lancet 390, 681–696 (2017).
Meri, S. Complement activation in diseases presenting with thrombotic microangiopathy. Eur. J. Intern. Med. 24, 496–502 (2013).
Noris, M., Mescia, F. & Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8, 622–633 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02205541 (2017).
Zipfel, P. F. et al. The role of complement in C3 glomerulopathy. Mol. Immunol. 67, 21–30 (2015).
Noris, M. & Remuzzi, G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and C3 glomerulopathy: core curriculum 2015. Am. J. Kidney Dis. 66, 359–375 (2015).
Nester, C. M. & Smith, R. J. Complement inhibition in C3 glomerulopathy. Semin. Immunol. 28, 241–249 (2016).
DeAngelis, R. A., Reis, E. S., Ricklin, D. & Lambris, J. D. Targeted complement inhibition as a promising strategy for preventing inflammatory complications in hemodialysis. Immunobiology 217, 1097–1105 (2012).
Fremeaux-Bacchi, V. & Legendre, C. M. The emerging role of complement inhibitors in transplantation. Kidney Int. 88, 967–973 (2015).
Damman, J. et al. Systemic complement activation in deceased donors is associated with acute rejection after renal transplantation in the recipient. Transplantation 92, 163–169 (2011).
Stegall, M. D., Chedid, M. F. & Cornell, L. D. The role of complement in antibody-mediated rejection in kidney transplantation. Nat. Rev. Nephrol. 8, 670–678 (2012).
Cooper, D. K., Ekser, B., Ramsoondar, J., Phelps, C. & Ayares, D. The role of genetically engineered pigs in xenotransplantation research. J. Pathol. 238, 288–299 (2016).
Flyvbjerg, A. The role of the complement system in diabetic nephropathy. Nat. Rev. Nephrol. 13, 311–318 (2017).
Fortpied, J., Vertommen, D. & Van Schaftingen, E. Binding of mannose-binding lectin to fructosamines: a potential link between hyperglycaemia and complement activation in diabetes. Diabetes Metab. Res. Rev. 26, 254–260 (2010).
Acosta, J. et al. Molecular basis for a link between complement and the vascular complications of diabetes. Proc. Natl Acad. Sci. USA 97, 5450–5455 (2000).
Phieler, J., Garcia-Martin, R., Lambris, J. D. & Chavakis, T. The role of the complement system in metabolic organs and metabolic diseases. Semin. Immunol. 25, 47–53 (2013).
Borne, Y. et al. Complement C3 associates with incidence of diabetes, but no evidence of a causal relationship. J. Clin. Endocrinol. Metab. http://dx.doi.org//10.1210/jc.2017-00948 (2017).
Lai, K. N. et al. IgA nephropathy. Nat. Rev. Dis. Primers 2, 16001 (2016).
Yu, F., Haas, M., Glassock, R. & Zhao, M. H. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 13, 483–495 (2017).
Sciascia, S. et al. Expanding the therapeutic options for renal involvement in lupus: eculizumab, available evidence. Rheumatol. Int. 37, 1249–1255 (2017).
Chen, M., Jayne, D. R. W. & Zhao, M. H. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat. Rev. Nephrol. 13, 359–367 (2017).
Schreiber, A. et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009). Elucidation of the involvement of complement in the pathology of AAV in this study provided an important starting point for the therapeutic use of C5aR1 antagonists in this disorder.
Frei, Y., Lambris, J. D. & Stockinger, B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol. Cell. Probes 1, 141–149 (1987). This paper describes the first monoclonal antibody against (murine) C5, thereby spearheading the development of therapeutic anti-C5 antibodies such as eculizumab.
Wurzner, R. et al. Inhibition of terminal complement complex formation and cell lysis by monoclonal antibodies. Complement Inflamm. 8, 328–340 (1991).
Wang, Y., Rollins, S. A., Madri, J. A. & Matis, L. A. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl Acad. Sci. USA 92, 8955–8959 (1995). This seminal proof-of-concept study demonstrates the therapeutic benefit of anti-C5 therapy.
Wang, H. et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J. Immunol. 179, 4451–4463 (2007).
Rinder, C. S. et al. Blockade of C5a and C5b-9 generation inhibits leukocyte and platelet activation during extracorporeal circulation. J. Clin. Invest. 96, 1564–1572 (1995).
Risitano, A. M. & Marotta, S. Therapeutic complement inhibition in complement-mediated hemolytic anemias: past, present and future. Semin. Immunol. 28, 223–240 (2016).
Hillmen, P. et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 162, 62–73 (2013). This study contributes to the regained confidence in complement-targeting strategies by providing further evidence of a beneficial safety profile of therapeutic complement inhibition at the level of C5 in a chronic disease setting.
Licht, C. et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 87, 1061–1073 (2015).
Ricklin, D., Barratt-Due, A. & Mollnes, T. E. Complement in clinical medicine: clinical trials, case reports and therapy monitoring. Mol. Immunol. 89, 10–21 (2017).
Alexion Pharmaceuticals. FDA approves Soliris® (eculizumab) for the treatment of patients with generalized myasthenia gravis (GMG). Alexion Pharmaceuticals http://news.alexion.com/press-release/product-news/fda-approves-soliris-eculizumab-treatment-patients-generalized-myasthenia (2017).
Alexion Pharmaceuticals. Alexion reports second quarter 2017 results. Alexion Pharmaceuticals http://news.alexionpharma.com/press-release/financial-news/alexion-reports-second-quarter-2017-results (2017).
Alexion Pharmaceuticals. New data from phase 3 REGAIN study of eculizumab (Soliris®) in patients with refractory generalized myasthenia gravis (GMG) presented at ICNMD annual congress. Alexion Pharmaceuticals http://news.alexionpharma.com/press-release/product-news/new-data-phase-3-regain-study-eculizumab-soliris-patients-refractory-gene (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01997229 (2017).
Coyle, D., Cheung, M. C. & Evans, G. A. Opportunity cost of funding drugs for rare diseases: the cost-effectiveness of eculizumab in paroxysmal nocturnal hemoglobinuria. Med. Decis. Making 34, 1016–1029 (2014).
Amgen. A randomized, double-blind, single-dose, 3-arm, parallel group study to determine the pharmacokinetic similarity of ABP 959 and eculizumab (Soliris®) in healthy male subjects. Australian New Zealand Clinical Trials Registry https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369851 (2016).
Nishimura, J. et al. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 370, 632–639 (2014).
Lin, Z. et al. Complement C3dg-mediated erythrophagocytosis: implications for paroxysmal nocturnal hemoglobinuria. Blood 126, 891–894 (2015).
Harder, M. J. et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood 129, 970–980 (2017). This landmark study provides the first mechanistic insight into the clinical observation that anti-C5 therapies can be exhausted under conditions of extensive complement activation, leading to pharmacodynamic breakthrough.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02534909 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02763644 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01526889 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02515942 (2017).
Fukuzawa, T. et al. Long lasting neutralization of C5 by SKY59, a novel recycling antibody, is a potential therapy for complement-mediated diseases. Sci. Rep. 7, 1080 (2017).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007).
Regeneron. Regeneron reports second quarter 2017 financial and operating results. Regeneron Pharmaceuticals http://newsroom.regeneron.com/releasedetail.cfm?ReleaseID=1035825 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03115996 (2017).
Adienne Pharma & Biotech. MUBODINA. Adienne http://www.adienne.com/rnd/molecules-in-development/mubodina (2017).
Ophthotech. Ophthotech expands focus with development for ophthalmic orphan diseases. Ophthotech http://investors.ophthotech.com/releasedetail.cfm?ReleaseID=1034437 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02397954 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02686658 (2017).
Nunn, M. A. et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J. Immunol. 174, 2084–2091 (2005).
Akari Therapeutics. Akari therapeutics demonstrates positive response with coversin in ongoing phase 2 PNH trial and in additional clinical targets. Akari Therapeutics http://akaritx.com/wp-content/uploads/2017/04/2017-04-24-Akari-announces-positive-Interim-Data-From-Phase-II-PNH-and-Corporate-Update.pdf (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02591862 (2017).
Kuhn, N., Schmidt, C. Q., Schlapschy, M. & Skerra, A. PASylated coversin, a C5-specific complement inhibitor with extended pharmacokinetics, shows enhanced anti-hemolytic activity in vitro. Bioconjug. Chem. 27, 2359–2371 (2016).
Berglund, M. M. & Stromberg, P. The clinical potential of affibody-based inhibitors of C5 for therapeutic complement disruption. Expert Rev. Proteomics 13, 241–243 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02083666 (2015).
Ricardo, A. et al. Preclinical evaluation of RA101495, a potent cyclic peptide inhibitor of C5 for the treatment of paroxysmal nocturnal hemoglobinuria [abstract]. Blood 126, 939 (2015).
Ra Pharma. Ra pharmaceuticals receives orphan drug designation from the U. S. FDA for RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria. Ra Pharma http://ir.rapharma.com/phoenix.zhtml?c=254447&p=irol-newsArticle&ID=2290097 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03078582 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03030183 (2017).
Keshari, R. S. et al. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc. Natl Acad. Sci. USA http://dx.doi.org/10.1073/pnas.1706818114 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02352493 (2017).
Alnylam Pharmaceuticals. Alnylam reports initial ALN-CC5 results in patients with paroxysmal nocturnal hemoglobinuria (PNH) from ongoing phase 1/2 study. Alnylam Pharmaceuticals http://investors.alnylam.com/releasedetail.cfm?ReleaseID=975341 (2016).
Hill, A. et al. A subcutaneously administered investigational RNAi therapeutic (ALN-CC5) targeting complement C5 for treatment of PNH and complement-mediated diseases: preliminary phase 1/2 study results in patients with PNH. Blood 128, 3891 (2016).
Alnylam Pharmaceuticals. Alnylam initiates phase 2 clinical study of cemdisiran (ALN-CC5) in patients with atypical hemolytic-uremic syndrome (aHUS). Alnylam Pharmaceuticals http://investors.alnylam.com/releasedetail.cfm?ReleaseID=1041647 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02949128 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02946463 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03056040 (2017).
Sheridan, D. et al. Design and preclinical characterization of ALXN1210: a next generation anti-C5 monoclonal antibody with improved pharmacokinetics and duration of action. Immunobiology 221, 1158 (2016).
Bomback, A. S. et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin. J. Am. Soc. Nephrol. 7, 748–756 (2012).
Zeerleder, S. C1-inhibitor: more than a serine protease inhibitor. Semin. Thromb. Hemost. 37, 362–374 (2011).
Nielsen, E. W. et al. Effect of supraphysiologic levels of C1-inhibitor on the classical, lectin and alternative pathways of complement. Mol. Immunol. 44, 1819–1826 (2007).
Chakradhar, S. The road less traveled: start-ups invest in novel approaches against neurodegeneration. Nat. Med. 22, 11–13 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02502903 (2017).
Bioverativ. Bioverativ completes acquisition of True North Therapeutics. Bioverativ https://www.bioverativ.com/newsroom/bioverativ-completes-acquisition-of-true-north-therapeutics.aspx (2017).
Fujita, T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2, 346–353 (2002).
Garred, P. et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol. Rev. 274, 74–97 (2016).
Schwaeble, W. J. et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc. Natl Acad. Sci. USA 108, 7523–7528 (2011).
Farrar, C. A., Zhou, W. & Sacks, S. H. Role of the lectin complement pathway in kidney transplantation. Immunobiology 221, 1068–1072 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02682407 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03205995 (2017).
Omeros. FDA grants orphan drug designation to Omeros' OMS721 for treatment of IgA nephropathy. Omeros http://investor.omeros.com/phoenix.zhtml?c=219263&p=irol-newsArticle_Print&id=2292002 (2017).
Boross, P., Yildiz, C., Simons, P. J., Boon, L. & Hack, C. E. A monoclonal antibody against complement C2, as a novel complement inhibitor. Altant Conference 2016: Innate Host Defence Utrecht, Netherlands (2016).
Ricklin, D. & Lambris, J. D. Therapeutic control of complement activation at the level of the central component C3. Immunobiology 221, 740–746 (2016).
Mastellos, D. C., Reis, E. S., Ricklin, D., Smith, R. J. & Lambris, J. D. Complement C3-targeted therapy: replacing long-held assertions with evidence-based discovery. Trends Immunol. 38, 383–394 (2017).
Maibaum, J. et al. Small-molecule factor D inhibitors targeting the alternative complement pathway. Nat. Chem. Biol. 12, 1105–1110 (2016). This paper describes the successful development of small molecule inhibitors of FD.
Vulpetti, A. et al. Structure-based library design and fragment screening for the identification of reversible complement factor D protease inhibitors. J. Med. Chem. 60, 1946–1958 (2017).
Yuan, X. et al. Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica 102, 466–475 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03053102 (2017).
Achillion Pharmaceuticals. Achillion reports second quarter 2017 financial results and proof-of-concept with a first-in-class, oral factor D inhibitor. Achillion Pharmaceuticals http://ir.achillion.com/releasedetail.cfm?ReleaseID=1036547 (2017).
Forneris, F. et al. Structures of C3b in complex with factors B and D give insight into complement convertase formation. Science 330, 1816–1820 (2010). This seminal study provides structural insight into the formation and functional determinants of the alternative pathway C3 convertase and facilitated the development of convertase-targeted therapeutics.
Katschke, K. J. Jr et al. Inhibiting alternative pathway complement activation by targeting the factor D exosite. J. Biol. Chem. 287, 12886–12892 (2012).
Loyet, K. M. et al. Complement inhibition in cynomolgus monkeys by anti-factor d antigen-binding fragment for the treatment of an advanced form of dry age-related macular degeneration. J. Pharmacol. Exp. Ther. 351, 527–537 (2014). In this important preclinical study of the anti-FD antibody lampalizumab, the researchers elucidate important aspects of local and systemic administration of a complement inhibitor considered for AMD treatment.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02288559 (2017).
Yaspan, B. L. et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci. Transl Med. 9, eaaf1443 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02247531 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02247479 (2017).
Genentech. Genentech provides update on first lampalizumab phase III study for geographic atrophy, an advanced form of age-related macular degeneration. Genentech https://www.gene.com/media/press-releases/14681/2017-09-08/genentech-provides-update-on-first-lampa (2017).
Irmscher, S. et al. Kallikrein represents an independent complement activator. Mol. Immunol. 89, 140–140 (2017).
Zhang, Y. Z. et al. Activation of alternative complement pathway without complement factor D. Mol. Immunol. 89, 173–173 (2017).
Omeros. Single dose of Omeros' lead MASP-3 inhibitor OMS906 shows multi-week blockade of the alternative pathway. Omeros http://investor.omeros.com/phoenix.zhtml?c=219263&p=irol-newsArticle_Print&id=2221301 (2016).
Dobo, J. et al. MASP-3 is the exclusive pro-factor D activator in resting blood: the lectin and the alternative complement pathways are fundamentally linked. Sci. Rep. 6, 31877 (2016).
Sekine, H., Takahashi, M., Iwaki, D. & Fujita, T. The role of MASP-1/3 in complement activation. Adv. Exp. Med. Biol. 735, 41–53 (2013).
McCaleb, M. et al. Systemic pharmacodynamic efficacy of a complement factor B antisense oligonucleotide in preclinical and phase 1 clinical studies. Invest. Ophthalmol. Visual Sci. 58, 1952 (2017).
Isis Pharmaceuticals. A masked, placebo-controlled, dose-escalation, phase 1 study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of ISIS 696844 administered subcutaneously to healthy volunteers. National Health Service https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/phase-1-sadmad-study-with-subcutaneous-isis-696844/ (2015).
Ionis Pharmaceuticals. Ionis to independently advance inotersen and IONIS-FB-L Rx. Ionis Pharmaceuticals http://ir.ionispharma.com/news-releases/news-release-details/ionis-independently-advance-inotersen-and-ionis-fb-l-rx (2017).
Lazar, H. L. et al. Beneficial effects of complement inhibition with soluble complement receptor 1 (TP10) during cardiac surgery: is there a gender difference? Circulation 116, I83–I88 (2007).
Zhang, Y. et al. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J. Am. Soc. Nephrol. 24, 1820–1829 (2013).
Risitano, A. M. et al. The complement receptor 2/factor H fusion protein TT30 protects paroxysmal nocturnal hemoglobinuria erythrocytes from complement-mediated hemolysis and C3 fragment. Blood 119, 6307–6316 (2012).
Smith, G. P. & Smith, R. A. Membrane-targeted complement inhibitors. Mol. Immunol. 38, 249–255 (2001).
Kassimatis, T. et al. A double-blind randomised controlled investigation into the efficacy of Mirococept (APT070) for preventing ischaemia reperfusion injury in the kidney allograft (EMPIRIKAL): study protocol for a randomised controlled trial. Trials 18, 255 (2017).
King's College London. EMPIRIKAL — a double blind randomized controlled investigation into the efficacy of Mirococept (APT070) for preventing ischaemia-reperfusion injury in the kidney allograft (EMPIRIKAL). National Health Service https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/empirikal/ (2013).
Schmidt, C. Q. et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J. Immunol. 190, 5712–5721 (2013).
Nilsson, P. H. et al. Autoregulation of thromboinflammation on biomaterial surfaces by a multicomponent therapeutic coating. Biomaterials 34, 985–994 (2013).
Wu, Y. Q. et al. Protection of nonself surfaces from complement attack by factor H-binding peptides: implications for therapeutic medicine. J. Immunol. 186, 4269–4277 (2011).
Sahu, A., Kay, B. K. & Lambris, J. D. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 157, 884–891 (1996). This paper provides the first description of C3 inhibitors of the compstatin family, of which several derivatives are currently in clinical development and evaluation.
Mastellos, D. C. et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur. J. Clin. Invest. 45, 423–440 (2015).
Katragadda, M., Magotti, P., Sfyroera, G. & Lambris, J. D. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 49, 4616–4622 (2006).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02264639 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02588833 (2017).
Apellis Pharmaceuticals. Positive data from APL-2 studies show rapid and durable improvements in LDH and hemoglobin levels in PNH. Apellis Pharmaceuticals http://www.apellis.com/pdfs/APL-2PressReleaseClinicalUpdateFINALv5-170629.pdf (2017).
Apellis Pharmaceuticals. Apellis pharmaceuticals announces that APL-2 met its primary endpoint in a phase 2 study in patients with geographic atrophy, an advanced form of age-related macular degeneration. Apellis Pharmaceuticals http://www.apellis.com/pdfs/Press%20Release%20FILLY%2012%20Month%20Results%20FINAL%20FINAL%20170823.pdf (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03226678 (2017).
Apellis Pharmaceuticals. Phase 1, double-blind, randomized, placebo-controlled, single ascending dose study of subcutaneous APL-9 in healthy volunteers. Apellis Pharmaceuticals https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370988 (2017).
Qu, H. et al. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology 218, 496–505 (2013).
Amyndas Pharmaceuticals. Amyndas' lead candidate AMY-101 receives orphan drug status from the FDA and the EMA for the treatment of C3 glomerulopathy. FiercePharma http://www.fiercepharma.com/press-releases/amyndas-lead-candidate-amy-101-receives-orphan-drug-status-fda-and-ema-trea (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03316521 (2017).
Hawksworth, O. A., Li, X. X., Coulthard, L. G., Wolvetang, E. J. & Woodruff, T. M. New concepts on the therapeutic control of complement anaphylatoxin receptors. Mol. Immunol. 89, 36–43 (2017).
Cukier-Meisner, E. Complementary ALS play. Biocentury https://www.biocentury.com/biocentury/emerging-company-profile/2017-07-11/why-alsonex-targeting-complement-system-c5a-treat-als (2017).
Finch, A. M. et al. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 42, 1965–1974 (1999).
Xiao, H. et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J. Am. Soc. Nephrol. 25, 225–231 (2014).
Jayne, D. R. et al. Randomized trial of C5a receptor inhibitor Avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 2756–2767 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02994927 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02222155 (2016).
ChemoCentryx. ChemoCentryx reports second quarter 2017 financial results and recent highlights. ChemoCentryx http://ir.chemocentryx.com/releasedetail.cfm?ReleaseID=1036588 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02464891 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02384317 (2016).
Moriconi, A. et al. Targeting the minor pocket of C5aR for the rational design of an oral allosteric inhibitor for inflammatory and neuropathic pain relief. Proc. Natl Acad. Sci. USA 111, 16937–16942 (2014).
Innate Pharma. Innate Pharma strengthens its proprietary pipeline with the acquisition of anti-C5aR, a first-in-class clinical-stage antibody, from novo Nordisk A/S. Innate Pharma http://www.innate-pharma.com/en/news-events/press-releases/innate-pharma-strengthens-its-proprietary-pipeline-acquisition-anti-c5ar-first-class-clinical-stage-antibodyfrom-novo-nordisk/s (2017).
Riedemann, N. C. et al. Controlling the anaphylatoxin C5a in diseases requires a specifically targeted inhibition. Clin. Immunol. 180, 25–32 (2017).
InflaRx. InflaRx Reports Topline Phase IIa Clinical Results of IFX-1 for the Treatment of Hidradenitis Suppurativa. InflaRx http://www.inflarx.de/Home/Investors/Press-Releases/09-2017--InflaRx-Reports-Topline-Phase-IIa-Clinical-Results-of-IFX-1-for-the-Treatment-of-Hidradenitis-Suppurativa0.html (2017).
InflaRx. The InflaRx technology. InflaRx http://www.inflarx.de/Home/Research---Development/Technology.html (2017).
Morgan, B. P. The membrane attack complex as an inflammatory trigger. Immunobiology 221, 747–751 (2016).
Taylor, R. P. & Lindorfer, M. A. Cytotoxic mechanisms of immunotherapy: harnessing complement in the action of anti-tumor monoclonal antibodies. Semin. Immunol. 28, 309–316 (2016).
Cassia, M., Alberici, F., Gallieni, M. & Jayne, D. Lupus nephritis and B-cell targeting therapy. Expert Rev. Clin. Immunol. 13, 951–962 (2017).
de Jong, R. N. et al. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol. 14, e1002344 (2016).
Diebolder, C. A. et al. Complement is activated by IgG hexamers assembled at the cell surface. Science 343, 1260–1263 (2014). In this landmark paper, the researchers describe the structural base of antibody recognition by complement on cell surfaces; their findings have important implications for the design of therapeutic antibodies that induce complement-mediated cytotoxicity.
Gros, P., Milder, F. J. & Janssen, B. J. Complement driven by conformational changes. Nat. Rev. Immunol. 8, 48–58 (2008).
Primikyri, A. et al. Method development and validation for the quantitation of the complement inhibitor Cp40 in human and cynomolgus monkey plasma by UPLC-ESI-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1041–1042, 19–26 (2017).
Risitano, A. M. et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood 123, 2094–2101 (2014).
Reis, E. S. et al. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology 220, 476–482 (2015).
Audemard-Verger, A. et al. Infections revealing complement deficiency in adults: a french nationwide study enrolling 41 patients. Medicine (Baltimore) 95, e3548 (2016).
Reis, E. S., Falcao, D. A. & Isaac, L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 63, 155–168 (2006).
Lachmann, P. J. & Smith, R. A. Taking complement to the clinic — has the time finally come? Scand. J. Immunol. 69, 471–478 (2009).
McNamara, L. A. et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb. Mortal. Wkly. Rep. 66, 734–737 (2017).
Konar, M. & Granoff, D. M. Eculizumab treatment and impaired opsonophagocytic killing of meningococci by whole blood from immunized adults. Blood 130, 891–899 (2017). This article provides insight into the mechanisms by which neisserial infections might occur in vaccinated patients receiving anti-C5 treatment and specifically into why targeting alternative pathway activation might confer advantages in this context.
Wang, J. et al. Using an in vitro xenoantibody-mediated complement-dependent cytotoxicity model to evaluate the complement inhibitory activity of the peptidic C3 inhibitor Cp40. Clin. Immunol. 162, 37–44 (2016).
Egan, M., Sullivan, K., Frazer-Abel, A. & Cunningham-Rundles, C. A healthy female with C3 hypocomplementemia and C3 Nephritic Factor. Clin. Immunol. 169, 14–15 (2016).
Gewurz, A. T., Imherr, S. M., Strauss, S., Gewurz, H. & Mold, C. C3 nephritic factor and hypocomplementaemia in a clinically healthy individual. Clin. Exp. Immunol. 54, 253–258 (1983).
Sfyroera, G. et al. Rare loss-of-function mutation in complement component C3 provides insight into molecular and pathophysiological determinants of complement activity. J. Immunol. 194, 3305–3316 (2015).
Hajishengallis, G. et al. Complement inhibition in pre-clinical models of periodontitis and prospects for clinical application. Semin. Immunol. 28, 285–291 (2016).
Maekawa, T. et al. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J. Clin. Periodontol. 43, 238–249 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01229215 (2016).
Thurman, J. M. & Nester, C. M. All things complement. Clin. J. Am. Soc. Nephrol. 11, 1 856–1866 (2016).
Sahutoglu, T. et al. Can eculizumab be discontinued in aHUS? Case report and review of the literature. Medicine (Baltimore) 95, e4330 (2016).
Nester, C. M. et al. Atypical aHUS: state of the art. Mol. Immunol. 67, 31–42 (2015).
Omeros. Omeros to present results from dose-ranging stage of OMS721 clinical trial in atypical hemolytic uremic syndrome at World Congress of Nephrology. Omeros http://investor.omeros.com/phoenix.zhtml?c=219263&p=irol-newsArticle&id=2256515 (2017).
Zhang, Y. et al. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology 220, 993–998 (2015).
ChemoCentryx. ChemoCentryx reports improvement in renal physiology and stabilization of kidney function following treatment with orally administered complement inhibitor CCX168 (Avacopan) in patient with fefractory C3 glomerulopathy. ChemoCentryx http://ir.chemocentryx.com/releasedetail.cfm?ReleaseID=995869 (2016).
Johnson, C. K. & Leca, N. Eculizumab use in kidney transplantation. Curr. Opin. Organ Transplant. 20, 643–651 (2015).
Jager, N. M., Poppelaars, F., Daha, M. R. & Seelen, M. A. Complement in renal transplantation: the road to translation. Mol. Immunol. 89, 22–35 (2017).
Stegall, M. D. et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am. J. Transplant. 11, 2405–2413 (2011).
Cornell, L. D., Schinstock, C. A., Gandhi, M. J., Kremers, W. K. & Stegall, M. D. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am. J. Transplant. 15, 1293–1302 (2015).
Alexion Pharmaceuticals. Alexion provides update on phase 2 clinical trial with eculizumab in antibody mediated rejection (AMR) in living-donor kidney transplant recipients. Alexion Pharmaceuticals http://news.alexionpharma.com/press-release/company-news/alexion-provides-update-phase-2-clinical-trial-eculizumab-antibody-mediat (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01895127 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01399593 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02222545 (2017).
Amyndas Pharmaceuticals. Clinical trials. Amyndas Pharmaceuticals http://amyndas.com/clinical-trials/ (2017).
US Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1492422/000119312515342824/d23325ds1.htm (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02461771 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02503332 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02745119 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02598583 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02605993 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02878616 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01527500 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03157635 (2017).
Clinical Research Register. Clinicaltrialsregister.ru http://clinicaltrialsregister.ru/search/index1.php?q=ECU-PNH-I&s= (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02245412 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02128269 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02246595 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02866825 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03001622 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02435732 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02547220 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01275976 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02251041 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02134314 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02936479 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02869347 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01303952 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02093533 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02493725 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01567085 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01919346 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02145182 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01106027 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02301624 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01892345 (2017).
Acknowledgements
The authors' work was supported by grants from the US National Institutes of Health (AI068730, AI030040) and the US National Science Foundation (grant No. 1423304), from the Swiss National Science Foundation (grant 31003A_176104) and by funding from the European Community's Seventh Framework Programme, under grant agreement number 602699 (DIREKT).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, contributed to discussions of the content, wrote the text and reviewed or edited the article before submission.
Corresponding authors
Ethics declarations
Competing interests
J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors (including third-generation compstatin analogues such as AMY-101). J.D.L. and D.R. are inventors of patents or patent applications that describe the use of complement inhibitors for therapeutic purposes, some of which are developed by Amyndas Pharmaceuticals. J.D.L. is also the inventor of the compstatin technology licensed to Apellis Pharmaceuticals (4(1MeW)7W, also known as POT-4 and APL-1) and pegylated derivatives such as APL-2. D.R. has received speaker's honoraria from Alexion and Novartis. The other authors declare no competing interests.
Glossary
- Immunoediting
-
Complement-mediated immunoediting is a tightly regulated process by which complement tags (opsonizes) and subsequently spares or eliminates host cells, depending on the magnitude and quality of complement responses in distinct pathophysiological contexts. This process might involve other immune-related mechanisms and influences cell-specific or tissue-specific homeostatic and developmental pathways (for example, cell lineage commitment or turnover during development) as well as tissue regeneration and repair.
- Synaptic pruning
-
The selective elimination of excess or unwanted synapses in the central nervous system during normal brain development and in age-related progressive neurological disorders. This process is mainly mediated by brain mononuclear phagocytic cells (that is, microglia) and reactive astrocytes.
- Accommodation
-
In transplantation medicine, accommodation describes an acquired resistance of a transplanted organ to damage caused by complement, antibodies and other immune effector mechanisms.
- Biosimilar
-
A biomedical product, such as a therapeutic antibody, that shares a high degree of structural and functional similarity with a product that is already clinically approved. Similar to small-molecule generic drugs, biosimilars are typically introduced once the patent protection of the original product expires.
- Extravascular haemolysis
-
The process by which erythrocytes are eliminated through phagocytosis in the spleen, liver or bone marrow.
- Pharmacodynamic breakthrough
-
A situation in which the inhibitory capacity of a complement-targeted therapeutic is exhausted owing to excessive complement activation, for example, as a result of acute infection or other triggers. Pharmacodynamic breakthrough might lead to a flare of disease symptoms despite continuous treatment.
- Minibody
-
An engineered antibody fragment consisting of two single-chain variable fragments linked by a dimer of CH3 (and sometimes also CH2) domains of an antibody.
- Aptamers
-
Short biopolymer sequences, typically of oligonucleotide (single-stranded RNA or DNA) or peptide origin, which are selected from a large, random sequence pool on the basis of their ability to bind a molecular target with high specificity.
- Affibody
-
A small engineered protein based on the protein A scaffold from Staphylococcus aureus. Combinatorial variation of key residues in the scaffold enables the generation of affibody proteins that mimic antibodies by binding various targets with high affinity.
- Macrocyclic peptide
-
Nonlinear peptides of natural or synthetic origin that form ring structures containing several peptide residues. Macrocyclic peptides often have distinct properties from their linear counterparts, and many are being developed as therapeutic inhibitors of protein–protein interactions.
- Exosite
-
In an enzyme, an exosite is a functionally important area of the protein that is distinct and often distant from the active or catalytic site. Exosites might, for example, mediate the binding of substrates or cofactors and might constitute interesting targets for inhibitors of protein–protein interactions, such as antibodies.
- Cytotopic drug
-
A drug that is targeted to and acts on a cell surface owing to a tethering moiety.
- HexaBodies
-
Engineered therapeutic antibodies with strong complement-mediated cytotoxic potential due to their increased propensity to form hexameric clusters on target surfaces such as cancer cells.
Rights and permissions
About this article
Cite this article
Ricklin, D., Mastellos, D., Reis, E. et al. The renaissance of complement therapeutics. Nat Rev Nephrol 14, 26–47 (2018). https://doi.org/10.1038/nrneph.2017.156
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.156
This article is cited by
-
Complement networks in gene-edited pig xenotransplantation: enhancing transplant success and addressing organ shortage
Journal of Translational Medicine (2024)
-
Systematic review of type 1 diabetes biomarkers reveals regulation in circulating proteins related to complement, lipid metabolism, and immune response
Clinical Proteomics (2023)
-
Complement therapeutics are coming of age in rheumatology
Nature Reviews Rheumatology (2023)
-
HLA class II antibody activation of endothelial cells induces M2 macrophage differentiation in peripheral blood
Clinical and Experimental Nephrology (2023)
-
Structure and function of a family of tick-derived complement inhibitors targeting properdin
Nature Communications (2022)