Key Points
-
Neural circuits that control immunity and inflammation provide novel targets for the treatment of kidney disease and hypertension
-
Activation of the cholinergic anti-inflammatory pathway (CAP) blocks splenic-dependent systemic inflammation and acute kidney injury (AKI); non-invasive, nonpharmacological approaches to activate the CAP include ultrasound application and vagus nerve stimulation
-
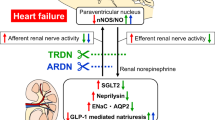
Neural circuits that directly regulate kidney function or carry sensory feedback from the kidney to the central nervous system could provide additional mechanisms for bidirectional neuroimmunomodulation of kidney disease
-
Interactions between neural circuits and the immune system have important roles in the pathogenesis of hypertension and renal fibrosis
-
Further defining neuroimmunomodulatory pathways in hypertension could enable the development of selective neuronal stimulatory or inhibitory strategies for lowering blood pressure that could potentially be more effective than renal denervation
-
Optogenetic tools provide unprecedented opportunities to dissect the neural pathways that control immunity and inflammation and enable the identification of novel approaches to therapy for kidney diseases
Abstract
Neural pathways regulate immunity and inflammation via the inflammatory reflex and specific molecular targets can be modulated by stimulating neurons. Neuroimmunomodulation by nonpharmacological methods is emerging as a novel therapeutic strategy for inflammatory diseases, including kidney diseases and hypertension. Electrical stimulation of vagus neurons or treatment with pulsed ultrasound activates the cholinergic anti-inflammatory pathway (CAP) and protects mice from acute kidney injury (AKI). Direct innervation of the kidney, by afferent and efferent neurons, might have a role in modulating and responding to inflammation in various diseases, either locally or by providing feedback to regions of the central nervous system that are important in the inflammatory reflex pathway. Increased sympathetic drive to the kidney has a role in the pathogenesis of hypertension, and selective modulation of neuroimmune interactions in the kidney could potentially be more effective for lowering blood pressure and treating inflammatory kidney diseases than renal denervation. Use of optogenetic tools for selective stimulation of specific neurons has enabled the identification of neural circuits in the brain that modulate kidney function via activation of the CAP. In this Review we discuss evidence for a role of neural circuits in the control of renal inflammation as well as the therapeutic potential of targeting these circuits in the settings of AKI, kidney fibrosis and hypertension.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Okusa, M. D., Chertow, G. M. & Portilla, D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin. J. Am. Soc. Nephrol. 4, 520–522 (2009).
Ojo, A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans. Am. Clin. Climatol Assoc. 125, 229–243; discussion 243–246 (2014).
Hsu, R. K., McCulloch, C. E., Dudley, R. A., Lo, L. J. & Hsu, C. Y. Temporal changes in incidence of dialysis-requiring AKI. J. Am. Soc. Nephrol. 24, 37–42 (2013).
Molitoris, B. A., Okusa, M. D., Palevsky, P. M., Kimmel, P. L. & Star, R. A. Designing clinical trials in acute kidney injury. Clin. J. Am. Soc. Nephrol. 7, 842–843 (2012).
Okusa, M. D. et al. Design of clinical trials in acute kidney injury: a report from an NIDDK workshop — prevention trials. Clin. J. Am. Soc. Nephrol. 7, 851–855 (2012).
Bonventre, J. V. et al. AKI: a path forward. Clin. J. Am. Soc. Nephrol. 8, 1606–1608 (2013).
Jo, S. K., Rosner, M. H. & Okusa, M. D. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin. J. Am. Soc. Nephrol. 2, 256–365 (2007).
Perrin, S. Preclinical research: make mouse studies work. Nature 507, 423–425 (2014).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). Study demonstrating an important and previously unrecognized anti-inflammatory role of the parasympathetic nervous system.
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Chiu, I. M., von Hehn, C. A. & Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067 (2012).
Ueno, M., Ueno-Nakamura, Y., Niehaus, J., Popovich, P. G. & Yoshida, Y. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat. Neurosci. 19, 784–787 (2016).
Inoue, T. et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J. Clin. Invest. 126, 1939–1952 (2016). Study demonstrating that VNS activates the CAP, resulting in activation of anti-inflammatory effects via α 7 nicotinic acetylcholine receptor–expressing splenic macrophages.
National Institutes of Health. Stimulating peripheral activity to relieve conditions. National Institutes of Health https://commonfund.nih.gov/SPARC (2017).
Ogbonnaya, S. & Kaliaperumal, C. Vagal nerve stimulator: evolving trends. J. Nat. Sci. Biol. Med. 4, 8–13 (2013).
Undem, B. J. & Kollarik, M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc. Am. Thorac Soc. 2, 355–360; discussion 371–372 (2005).
Chunchai, T. et al. Vagus nerve stimulation exerts the neuroprotective effects in obese-insulin resistant rats, leading to the improvement of cognitive function. Sci. Rep. 6, 26866 (2016).
De Ferrari, G. M. et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 32, 847–855 (2011).
Koopman, F. A. et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 113, 8284–8289 (2016). Pilot study demonstrating that VNS inhibited TNF production and improved disease severity in patients with rheumatoid arthritis.
Bonaz, B. et al. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. 28, 948–953 (2016). Pilot study demonstratating the potential efficacy and safety of chronic VNS in patients with Crohn disease.
Andersson, U. & Tracey, K. J. Neural reflexes in inflammation and immunity. J. Exp. Med. 209, 1057–1068 (2012).
Smith, K. A. Louis Pasteur, the father of immunology? Front. Immunol. 3, 68 (2012).
Blevins, S. M. & Bronze, M. S. Robert Koch and the 'golden age' of bacteriology. Int. J. Infect. Dis. 14, e744–e751 (2010).
Bernard, C. Leçons sur les phénomènes de la vie, communs aux animaux et aux végétaux [French]. Paris 307, (1878).
Thomas, L. Germs. N. Engl. J. Med. 287, 553–555 (1972).
Hotchkiss, R., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874 (2013).
Bellavance, M. A. & Rivest, S. The HPA — immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 5, 136 (2014).
Watkins, L. R. et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 183, 27–31 (1995). Seminal discovery that an intact vagus nerve is required for the development of fever in rodents following intra-abdominal administration of IL-1β. This finding demonstrates an essential early role for the sensory nervous system in a fundamental mechanism of host defence.
Niijima, A. The afferent discharges from sensors for interleukin 1β in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 61, 287–291 (1996).
Goehler, L. E. et al. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci. 85, 49–59 (2000).
Ordovas-Montanes, J. et al. The regulation of immunological processes by peripheral neurons in homeostasis and disease. Trends Immunol. 36, 578–604 (2015).
Pavlov, V. A. & Tracey, K. J. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci. 20, 156–166 (2017).
Ek, M., Kurosawa, M., Lundeberg, T. & Ericsson, A. Activation of vagal afferents after intravenous injection of interleukin-1β: role of endogenous prostaglandins. J. Neurosci. 18, 9471–9479 (1998).
Hermann, G. E. & Rogers, R. C. TNFα: a trigger of autonomic dysfunction. Neuroscientist 14, 53–67 (2008).
van der Kleij, H. et al. Evidence for neuronal expression of functional Fc (ε and γ) receptors. J. Allergy Clin. Immunol. 125, 757–760 (2010).
Page, A. J., O'Donnell, T. A. & Blackshaw, L. A. P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J. Physiol. 523, 403–411; part 2 (2000).
Brouns, I., Adriaensen, D., Burnstock, G. & Timmermans, J. P. Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X3 receptors. Am. J. Respir. Cell Mol. Biol. 23, 52–61 (2000).
Mascarucci, P., Perego, C., Terrazzino, S. & De Simoni, M. G. Glutamate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1β. Neuroscience 86, 1285–1290 (1998).
Ericsson, A., Kovacs, K. J. & Sawchenko, P. E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 14, 897–913 (1994).
Kalia, M. & Sullivan, J. M. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J. Comp. Neurol. 211, 248–265 (1982).
Bernik, T. R. et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195, 781–788 (2002).
van Westerloo, D. J. et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130, 1822–1830 (2006).
Ghia, J. E., Blennerhassett, P. & Collins, S. M. Vagus nerve integrity and experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G560–G567 (2007).
Ghia, J. E., Blennerhassett, P., Kumar-Ondiveeran, H., Verdu, E. F. & Collins, S. M. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130 (2006).
de Jonge, W. J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6, 844–851 (2005).
Andersson, U. & Tracey, K. J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 30, 313–335 (2012).
Tracey, K. J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296 (2007).
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
Huston, J. M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628 (2006).
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008).
Reilly, F. D., McCuskey, P. A., Miller, M. L., McCuskey, R. S. & Meineke, H. A. Innervation of the periarteriolar lymphatic sheath of the spleen. Tissue Cell 11, 121–126 (1979).
Bellinger, D. L., Lorton, D., Hamill, R. W., Felten, S. Y. & Felten, D. L. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun. 7, 191–204 (1993).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011). Landmark study demonstrating that an acetylcholine-producing, memory T cell population is integral to the inflammatory reflex following VNS in mice.
Franco, R., Pacheco, R., Lluis, C., Ahern, G. P. & O'Connell, P. J. The emergence of neurotransmitters as immune modulators. Trends Immunol. 28, 400–407 (2007).
Vida, G. et al. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 25, 4476–4485 (2011).
Wang, H. et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003).
Pena, G. et al. Unphosphorylated STAT3 modulates alpha7 nicotinic receptor signaling and cytokine production in sepsis. Eur. J. Immunol. 40, 2580–2589 (2010).
Metz, C. N. & Tracey, K. J. It takes nerve to dampen inflammation. Nat. Immunol. 6, 756–757 (2005).
Yeboah, M. M. et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 74, 62–69 (2008).
Chatterjee, P. K. et al. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS ONE 7, e35361 (2012).
Hoeger, S. et al. Modulation of brain dead induced inflammation by vagus nerve stimulation. Am. J. Transplant. 10, 477–489 (2010).
Hoeger, S. et al. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrol. Dial. Transplant. 29, 544–549 (2014).
Howland, R. H. Vagus nerve stimulation. Curr. Behav. Neurosci. Rep. 1, 64–73 (2014).
Ben-Menachem, E., Revesz, D., Simon, B. J. & Silberstein, S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur. J. Neurol. 22, 1260–1268 (2015).
Ay, I., Nasser, R., Simon, B. & Ay, H. Transcutaneous cervical vagus nerve stimulation ameliorates acute ischemic injury in rats. Brain Stimul. 9, 166–173 (2016).
Lerman, I. et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation 19, 283–290 (2016).
Gigliotti, J. C. & Okusa, M. D. The spleen: the forgotten organ in acute kidney injury of critical illness. Nephron Clin. Pract. 127, 153–157 (2014).
Gigliotti, J. C. et al. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J. Am. Soc. Nephrol. 24, 1451–1460 (2013). First study demonstrating that ultrasound treatment of mice prior to ischaemia–reperfusion injury protects kidney structure and function consistent with activation of the CAP.
Gigliotti, J. C. et al. Ultrasound modulates the splenic neuroimmune axis in attenuating AKI. J. Am. Soc. Nephrol. 26, 2407–2481 (2015).
Brimijoin, S. & Molinoff, P. B. Effects of 6-hydroxydopamine on the activity of tyrosine hydroxylase and dopamine-β-hydroxylase in sympathetic ganglia of the rat. J. Pharmacol. Exp. Ther. 178, 417–424 (1971).
Borovikova, L. V. et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton. Neurosci. 85, 141–147 (2000).
Pavlov, V. A. et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl Acad. Sci. USA 103, 5219–5223 (2006).
Li, H. Y., Ericsson, A. & Sawchenko, P. E. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc. Natl Acad. Sci. USA 93, 2359–2364 (1996).
Jansen, A. S., Nguyen, X. V., Karpitskiy, V., Mettenleiter, T. C. & Loewy, A. D. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270, 644–646 (1995).
Guyenet, P. G. et al. C1 neurons: the body's EMTs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R187–R204 (2013).
Abe, C. et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 20, 700–707 (2017). First demonstration that optogenetic stimulation of C1 neurons located in the medulla oblongata protects kidneys from ischaemia–reperfusion injury.
Nishi, E. E., Bergamaschi, C. T. & Campos, R. R. The crosstalk between the kidney and the central nervous system: the role of renal nerves in blood pressure regulation. Exp. Physiol. 100, 479–484 (2015).
Abdulla, M. H. & Johns, E. J. The innervation of the kidney in renal injury and inflammation: a cause and consequence of deranged cardiovascular control. Acta Physiol. (Oxf.) 220, 404–416 (2017).
Barajas, L., Liu, L. & Powers, K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can. J. Physiol. Pharmacol. 70, 735–749 (1992).
Burnstock, G. & Loesch, A. Sympathetic innervation of the kidney in health and disease: emphasis on the role of purinergic cotransmission. Auton. Neurosci. 204, 4–16 (2017).
DiBona, G. F. & Kopp, U. C. Neural control of renal function. Physiol. Rev. 77, 75–197 (1997). Comprehensive review of the role of the neural system in controlling renal function.
Ferguson, M., Ryan, G. B. & Bell, C. Localization of sympathetic and sensory neurons innervating the rat kidney. J. Auton. Nerv. Syst. 16, 279–288 (1986).
Ferguson, M. & Bell, C. Substance P-immunoreactive nerves in the rat kidney. Neurosci. Lett. 60, 183–188 (1985).
Kopp, U. C., Cicha, M. Z., Smith, L. A. & Hokfelt, T. Nitric oxide modulates renal sensory nerve fibers by mechanisms related to substance P receptor activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R279–R290 (2001).
Kopp, U. C., Olson, L. A. & DiBona, G. F. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am. J. Physiol. 246, F67–F77 (1984).
Kopp, U. C., Smith, L. A. & DiBona, G. F. Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am. J. Physiol. 249, F507–F517 (1985).
Johns, E. J. & Kopp, U. C. in Seldin and Giebisch: The Kidney: Physiology and Pathophysiology (eds Alpern, R. J., Moe, O. W. & Caplan, M.) 451–486 (Academic, 2013).
Ciriello, J. & Calaresu, F. R. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J. Auton. Nerv. Syst. 8, 273–285 (1983).
Wyss, J. M. & Donovan, M. K. A direct projection from the kidney to the brainstem. Brain Res. 298, 130–134 (1984).
Kopp, U. C. Role of renal sensory nerves in physiological and pathophysiological conditions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R79–R95 (2015).
Stella, A. & Zanchetti, A. Functional role of renal afferents. Physiol. Rev. 71, 659–682 (1991).
Hermes, S. M., Colbert, J. F. & Aicher, S. A. Differential content of vesicular glutamate transporters in subsets of vagal afferents projecting to the nucleus tractus solitarii in the rat. J. Comp. Neurol. 522, 642–653 (2014).
Chang, R. B., Strochlic, D. E., Williams, E. K., Umans, B. D. & Liberles, S. D. Vagal sensory neuron subtypes that differentially control breathing. Cell 161, 622–633 (2015).
Ditting, T. et al. Tonic postganglionic sympathetic inhibition induced by afferent renal nerves? Hypertension 59, 467–476 (2012).
Norvell, J. E. & Anderson, J. M. Assessment of possible parasympathetic innervation of the kidney. J. Auton. Nerv. Syst. 8, 291–294 (1983).
Gattone, V. H. 2nd, Marfurt, C. F. & Dallie, S. Extrinsic innervation of the rat kidney: a retrograde tracing study. Am. J. Physiol. 250, F189–F196 (1986).
Weiss, M. L. & Chowdhury, S. I. The renal afferent pathways in the rat: a pseudorabies virus study. Brain Res. 812, 227–241 (1998).
Zheng, H. & Patel, K. P. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton. Neurosci. 204, 57–64 (2016).
Kim, J. & Padanilam, B. J. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J. Am. Soc. Nephrol. 24, 229–242 (2013). Study showing that renal denervation prevents fibrogenesis and the inflammatory cascade following ureteral obstruction.
Kim, J. & Padanilam, B. J. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 87, 350–358 (2015).
Banek, C. T. et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68, 1415–1423 (2016).
Foss, J. D., Wainford, R. D., Engeland, W. C., Fink, G. D. & Osborn, J. W. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R112–R122 (2015).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 371, 58–66 (2014). A summmary of the bidirectional relationship between AKI and CKD.
Basile, D. P. et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 27, 687–697 (2016).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 26, 1765–1776 (2015).
Basile, D. P., Donohoe, D., Roethe, K. & Osborn, J. L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 281, F887–F899 (2001).
Mimura, I. & Nangaku, M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 6, 667–678 (2010).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Lee, S. et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326 (2011).
Cao, Q., Harris, D. C. & Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology 30, 183–194 (2015).
Jang, H. R. & Rabb, H. Immune cells in experimental acute kidney injury. Nat. Rev. Nephrol. 11, 88–101 (2015).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Szeto, H. H. et al. Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1β and IL-18 and arrests CKD. J. Am. Soc. Nephrol. 28, 1437–1449 (2016).
Lovisa, S. et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 21, 998–1009 (2015).
Grande, M. T. et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 21, 989–997 (2015).
Perry, H. M. et al. Endothelial sphingosine 1phosphate receptor1 mediates protection and recovery from acute kidney injury. J. Am. Soc. Nephrol. 27, 3383–3393 (2016).
Venner, J. M., Famulski, K. S., Reeve, J., Chang, J. & Halloran, P. F. Relationships among injury, fibrosis, and time in human kidney transplants. JCI Insight 1, e85323 (2016).
Dampney, R. A. Central neural control of the cardiovascular system: current perspectives. Adv. Physiol. Educ. 40, 283–296 (2016).
Guyenet, P. G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346 (2006).
Joyner, M. J., Charkoudian, N. & Wallin, B. G. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56, 10–16 (2010).
Coffman, T. M. The inextricable role of the kidney in hypertension. J. Clin. Invest. 124, 2341–2347 (2014).
DiBona, G. F. & Esler, M. Translational medicine: the antihypertensive effect of renal denervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R245–R253 (2010).
Wenzel, U. et al. Immune mechanisms in arterial hypertension. J. Am. Soc. Nephrol. 27, 677–686 (2016).
Harrison, D. G. The immune system in hypertension. Trans. Am. Clin. Climatol Assoc. 125, 130–138; discussion 138–140 (2014).
Renaudin, C., Bataillard, A. & Sassard, J. Partial transfer of genetic hypertension by lymphoid cells in Lyon rats. J. Hypertens. 13, 1589–1592 (1995).
Okuda, T. & Grollman, A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex. Rep. Biol. Med. 25, 257–264 (1967).
White, F. N. & Grollman, A. Autoimmune factors associated with infarction of the kidney. Nephron 1, 93–102 (1964).
Dzielak, D. J. Immune mechanisms in experimental and essential hypertension. Am. J. Physiol. 260, R459–R467 (1991).
Bendich, A., Belisle, E. H. & Strausser, H. R. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem. Biophys. Res. Commun. 99, 600–607 (1981).
Guzik, T. J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007). Study demonstrating a role of T cells in the genesis of hypertension. This finding supports a role of inflammation in the pathogenesis of hypertension.
Tesmer, L. A., Lundy, S. K., Sarkar, S. & Fox, D. A. Th17 cells in human disease. Immunol. Rev. 223, 87–113 (2008).
Madhur, M. S. et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55, 500–507 (2010).
Schrader, L. I., Kinzenbaw, D. A., Johnson, A. W., Faraci, F. M. & Didion, S. P. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler. Thromb. Vasc. Biol. 27, 2576–2581 (2007).
Lee, D. L. et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 290, H935–H940 (2006).
Brands, M. W. et al. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 56, 879–884 (2010).
Venegas-Pont, M. et al. Tumor necrosis factor-α antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56, 643–649 (2010).
Tran, L. T., MacLeod, K. M. & McNeill, J. H. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol. Cell Biochem. 330, 219–228 (2009).
Bautista, L. E., Vera, L. M., Arenas, I. A. & Gamarra, G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J. Hum. Hypertens. 19, 149–154 (2005).
Herrera, J., Ferrebuz, A., MacGregor, E. G. & Rodriguez-Iturbe, B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 17, S218–S225 (2006).
Touyz, R. M. The neuroimmune axis in the kidney: role in hypertension. Circ. Res. 117, 487–489 (2015).
Burne, M. J. et al. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Invest. 108, 1283–1290 (2001). The seminal observation that CD4+ T cells are important mediators of ischaemic acute renal failure, which has led to therapeutic approaches to block T cells in the pathogenesis of AKI.
Li, L. et al. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J. Immunol. 178, 5899–5911 (2007).
Day, Y.-J. et al. Renal ischemia-reperfusion and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and inteferon-γ J. Immunol. 176, 3108–3114 (2006).
Zhang, J. & Crowley, S. D. Role of T lymphocytes in hypertension. Curr. Opin. Pharmacol. 21, 14–19 (2015).
Rodriguez-Iturbe, B., Pons, H. & Johnson, R. J. Role of the immune system in hypertension. Physiol. Rev. 97, 1127–1164 (2017).
Xiao, L. et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ. Res. 117, 547–557 (2015).
Carnevale, D. et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat. Commun. 7, 13035 (2016).
Case, A. J. & Zimmerman, M. C. Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J. Physiol. 594, 527–536 (2016).
Rumantir, M. S. et al. Neural mechanisms in human obesity-related hypertension. J. Hypertens. 17, 1125–1133 (1999).
Esler, M. et al. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol. Rev. 70, 963–985 (1990).
Esler, M. et al. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11, 3–20 (1988).
Krum, H. et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373, 1275–1281 (2009). This proof-of-principle trial in patients with resistant hypertension demonstrated that renal denervation decreased blood pressure without serious adverse events.
Esler, M. D. et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur. Heart J. 35, 1752–1759 (2014).
Katholi, R. E. Renal nerves and hypertension: an update. Fed. Proc. 44, 2846–2850 (1985).
Katholi, R. E. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am. J. Physiol. 245, F1–F14 (1983).
Campese, V. M. & Kogosov, E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 25, 878–882 (1995).
Converse, R. L. Jr. et al. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 327, 1912–1918 (1992).
Bhatt, D. L. et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 370, 1393–1401 (2014). The prospective, single-blind, randomized, sham-controlled SYMPLICITY HTN-3 trial showed that radiofrequency renal denervation did not significantly reduce systolic blood pressure in patients with resistant hypertension at 6 months.
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Gomez, J. L. et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017).
Cai, D., Cohen, K. B., Luo, T., Lichtman, J. W. & Sanes, J. R. Improved tools for the Brainbow toolbox. Nat. Methods 10, 540–547 (2013).
Koopman, F. A., Schuurman, P. R., Vervoordeldonk, M. J. & Tak, P. P. Vagus nerve stimulation: a new bioelectronics approach to treat rheumatoid arthritis? Best Pract. Res. Clin. Rheumatol 28, 625–635 (2014).
Matteoli, G. & Boeckxstaens, G. E. The vagal innervation of the gut and immune homeostasis. Gut 62, 1214–1222 (2013).
Clayton, S. C., Haack, K. K. & Zucker, I. H. Renal denervation modulates angiotensin receptor expression in the renal cortex of rabbits with chronic heart failure. Am. J. Physiol. Renal Physiol. 300, F31–F39 (2011).
Hering, D. et al. Renal denervation in moderate to severe CKD. J. Am. Soc. Nephrol. 23, 1250–1257 (2012).
Mahfoud, F. et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 60, 419–424 (2012).
Acknowledgements
The authors' research summarized in this review was supported by the NIH under awards 1R01DK105133, R01 DK062324, U18EB021787 and 1S10RR026799-01.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, made substantial contributions to discussion of the content, wrote the article and edited or reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.D.O. and D.L.R. own equity in Adenosine Therapeutics, LLC. K.J.T. is a consultant for SetPoint Medical, Inc.
Glossary
- Vagus nerve
-
The vagus or 10th cranial nerve is a pair of nerve bundles (right and left) that contains axons of both efferent and afferent neurons. The efferent neurons provide cholinergic input to almost all organs in the periphery and some skeletal muscles. Their cell bodies are located in the medulla oblongata. The cell bodies of afferent neurons are located in the nodose ganglia. These neurons carry the majority of sensory information from visceral organs to the CNS.
- Subdiaphragmatic vagotomy
-
Denervation by surgical transection of both trunks of the vagus nerve either below or caudal to the diaphragm.
- Nucleus tractus solitarius
-
A group of neuronal cell bodies in the brainstem that form an integrative centre for sensory information from the vagus, glossopharyngeal and facial nerves. The nucleus tractus solitarius projects to a wide variety of brain regions, including the hypothalamus, thalamus, and medulla, and participates in circuits that regulate autonomic function.
- Coeliac ganglion
-
A cluster of nerve cells in the abdomen that forms part of the prevertebral sympathetic chain and provides sympathetic innervation to most of the digestive tract. The coeliac ganglion is regulated by the preganglionic neurons in the intermediolateral cell column of the spinal cord.
- Bilateral cervical vagotomy
-
Bilateral cervical vagotomy is a surgical transection of the vagus nerve at the level of the neck on both sides. This procedure prevents the flow of information through the nerve in both directions (afferent and efferent) between its origin in the CNS and its targets in the periphery.
- Retrograde tract tracing
-
A technique for identifying neuronal pathways that exploits constitutive axonal transport to trace the movement of specialized proteins, markers or viruses (which can be visualized by various means) from their point of exogenous introduction in a target field of interest (synaptic terminals) to the cell bodies of those axons (retrograde transport). By contrast, anterograde tracing traces the transport of markers from the cell body region in the direction of the synapse.
Rights and permissions
About this article
Cite this article
Okusa, M., Rosin, D. & Tracey, K. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol 13, 669–680 (2017). https://doi.org/10.1038/nrneph.2017.132
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.132
This article is cited by
-
Interorgan communication networks in the kidney–lung axis
Nature Reviews Nephrology (2024)
-
Somatosensory cortex and central amygdala regulate neuropathic pain-mediated peripheral immune response via vagal projections to the spleen
Nature Neuroscience (2024)
-
A feasibility study on AI-controlled closed-loop electrical stimulation implants
Scientific Reports (2023)
-
Alpha 7 nicotinic acetylcholine receptors signaling boosts cell-cell interactions in macrophages effecting anti-inflammatory and organ protection
Communications Biology (2023)
-
Emerging topics on renal denervation in hypertension: anatomical and functional aspects of renal nerves
Hypertension Research (2023)