Key Points
-
Clinicians are now able to identify which patients with autosomal dominant polycystic kidney disease (ADPKD) are at highest risk of progression and most likely to benefit from early therapy
-
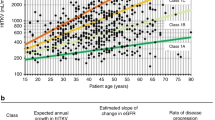
MRI measurement of total kidney volume (TKV) is a valuable method to predict future rate of increase in TKV, rate of decline in kidney function and risk of end-stage renal disease (ESRD)
-
The availability of genetic testing will continue to increase and can provide a diagnosis in unusual or atypical cases or in young (<30 years of age) patients being assessed for kidney donation
-
ADPKD is typically the result of germline PKD1 or PKD2 mutations, with somatic mutations, genetic mosaicism and modifier mutations occasionally contributing to the ADPKD phenotype
-
Disruption of polycystin trafficking and signalling, environmental exposures and the compounding 'snowball' effects of regional ischaemia, inflammation and tubular obstruction further contribute to disease progression
-
Novel strategies intended to limit cyst burden have provided encouraging results, whereas treatment of hypertension and proteinuria remain the mainstays of medical management of ADPKD
-
Assessment and treatment of ADPKD-related complications, including cyst haemorrhage, cyst infection, nephrolithiasis and chronic pain, require special consideration and attention
Abstract
The natural history of autosomal dominant polycystic kidney disease (ADPKD) is characterized by a variable rate of cyst development and increase in total kidney volume (TKV), variable kidney function decline and age of onset of end-stage renal disease (ESRD), and variable presentation of renal and extrarenal manifestations. Precision medicine is proposed to improve patient outcomes by tailoring therapy to the specific genetic background, pathophysiology, disease burden, prognosis and status of each individual. This approach to the management of patients with ADPKD is nearing clinical implementation owing to advances in genetics, imaging, biomarker development and therapeutics. In this Review, we discuss pharmacological and non-pharmacological interventions for the treatment of hypertension and proteinuria, and for slowing the rate of cyst growth in patients with ADPKD before the development of ESRD. We provide recommendations for the management of renal complications, including cyst infection, nephrolithiasis, haematuria and chronic pain. The early treatment of patients with ADPKD who are largely asymptomatic is associated with a therapeutic burden but slows cyst growth and delays subsequent loss of kidney function, which ultimately delays the need for renal replacement therapy and has a positive effect on the quality of life of patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gabow, P. A. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 329, 332–342 (1993).
Reule, S. et al. ESRD from autosomal dominant polycystic kidney disease in the United States, 2001–2010. Am. J. Kidney Dis. 64, 592–599 (2014).
Spithoven, E. M. et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival — an analysis of data from the ERA-EDTA Registry. Nephrol. Dial. Transplant. 29, iv15–iv25 (2014).
Fernando, M. R., Dent, H., McDonald, S. P. & Rangan, G. K. Incidence and survival of end-stage kidney disease due to polycystic kidney disease in Australia and New Zealand. Popul. Health Metr. 15, 7 (2017).
Chapman, A. B. et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 64, 1035–1045 (2003).
Schrier, R. W. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 (2014).
Torres, V. E. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 367, 2407–2418 (2012).
Torres, V. E. et al. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfx043 (2017).
Committee on A Framework for Developing a New Taxonomy of Disease. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. (The National Academies Press, 2011).
Harris, P. C. & Torres, V. E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Invest. 124, 2315–2324 (2014).
Cornec- Le Gall, E., Audrezet, M. P., Le Meur, Y., Chen, J. M. & Ferec, C. Genetics and pathogenesis of autosomal dominant polycystic kidney disease: 20 years on. Hum. Mutat. 35, 1393–1406 (2014).
Besse, W. et al. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J. Clin. Invest. 127, 1772–1785 (2017).
Lantinga-van Leeuwen, I. S. et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum. Mol. Genet. 13, 3069–3077 (2004).
Rowe, I. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 (2013).
Wang, X., Wu, Y., Ward, C. J., Harris, P. C. & Torres, V. E. Vasopressin directly regulates cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 102–108 (2008).
Takakura, A. et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum. Mol. Genet. 18, 2523–2531 (2009).
Leonhard, W. N. et al. Scattered deletion of PKD1 in kidneys causes a cystic snowball effect and recapitulates polycystic kidney disease. J. Am. Soc. Nephrol. 26, 1322–1333 (2015).
Houle, D., Govindaraju, D. R. & Omholt, S. Phenomics: the next challenge. Nat. Rev. Genet. 11, 855–866 (2010).
Lanktree, M. B., Hassell, R. G., Lahiry, P. & Hegele, R. A. Phenomics: expanding the role of clinical evaluation in genomic studies. J. Investig. Med. 58, 700–706 (2010).
Grantham, J. J. et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 73, 108–116 (2008).
Irazabal, M. V. et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 26, 160–172 (2015).
Gansevoort, R. T. et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol. Dial. Transplant. 31, 337–348 (2016).
Grantham, J. J. Rationale for early treatment of polycystic kidney disease. Pediatr. Nephrol. 30, 1053–1062 (2015).
Cornec-Le Gall, E. et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 942–951 (2016).
Schrier, R. W. et al. Predictors of autosomal dominant polycystic kidney disease progression. J. Am. Soc. Nephrol. 25, 2399–2418 (2014).
Hwang, Y. H. et al. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 1861–1868 (2016).
Heyer, C. M. et al. Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 2872–2884 (2016).
Cornec-Le Gall, E. et al. Type of PKD1 mutation influences renal outcome in ADPKD. J. Am. Soc. Nephrol. 24, 1006–1013 (2013).
Iliuta, I. A. et al. Polycystic kidney disease without an apparent family history. J. Am. Soc. Nephrol. 28, 2768–2776 (2017).
Barua, M. et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J. Am. Soc. Nephrol. 20, 1833–1838 (2009).
Porath, B. et al. Mutations in GANAB, encoding the glucosidase IIα subunit, cause autosomal-dominant polycystic kidney and liver disease. Am. J. Hum. Genet. 98, 1193–1207 (2016).
Braun, D. A. & Hildebrandt, F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 9, a028191 (2017).
Song, X., Haghighi, A., Iliuta, I. A. & Pei, Y. Molecular diagnosis of autosomal dominant polycystic kidney disease. Expert Rev. Mol. Diagn. 13, 1–11 (2017).
Kinoshita, M. et al. Technical evaluation: identification of pathogenic mutations in PKD1 and PKD2 in patients with autosomal dominant polycystic kidney disease by next-generation sequencing and use of a comprehensive new classification system. PLoS ONE 11, e0166288 (2016).
Mallawaarachchi, A. C. et al. Whole-genome sequencing overcomes pseudogene homology to diagnose autosomal dominant polycystic kidney disease. Eur. J. Hum. Genet. 24, 1584–1590 (2016).
Bergmann, C. et al. Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J. Am. Soc. Nephrol. 22, 2047–2056 (2011).
Cnossen, W. R. et al. LRP5 variants may contribute to ADPKD. Eur. J. Hum. Genet. 24, 237–242 (2016).
Rossetti, S. et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 75, 848–855 (2009).
Losekoot, M. et al. Neonatal onset autosomal dominant polycystic kidney disease (ADPKD) in a patient homozygous for a PKD2 missense mutation due to uniparental disomy. J. Med. Genet. 49, 37–40 (2012).
Ward, C. J. et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat. Genet. 30, 259–269 (2002).
Soroka, S. et al. Assessing risk of disease progression and pharmacological management of autosomal dominant polycystic kidney disease: a canadian expert consensus. Can. J. Kidney Health Dis. 4, 2054358117695784 (2017).
Pei, Y. et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 20, 205–212 (2009).
Tangri, N. et al. Total kidney volume as a biomarker of disease progression in autosomal dominant polycystic kidney disease. Can. J. Kidney Health Dis. 4, 2054358117693355 (2017).
Turco, D., Busutti, M., Mignani, R., Magistroni, R. & Corsi, C. Comparison of total kidney volume quantification methods in autosomal dominant polycystic disease for a comprehensive disease assessment. Am. J. Nephrol. 45, 373–379 (2017).
Grantham, J. J. & Torres, V. E. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat. Rev. Nephrol. 12, 667–677 (2016).
Grantham, J. J., Chapman, A. B. & Torres, V. E. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin. J. Am. Soc. Nephrol. 1, 148–157 (2006).
Grantham, J. J. et al. Volume progression in polycystic kidney disease. N. Engl. J. Med. 354, 2122–2130 (2006).
Cadnapaphornchai, M. A. et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 9, 889–896 (2014).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Torres, V. E. et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2267–2276 (2014).
Caroli, A. et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet 382, 1485–1495 (2013).
Stallone, G. et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol. Dial. Transplant. 27, 3560–3567 (2012).
Hogan, M. C. et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J. Am. Soc. Nephrol. 21, 1052–1061 (2010).
Braun, W. E., Schold, J. D., Stephany, B. R., Spirko, R. A. & Herts, B. R. Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study. Clin. J. Am. Soc. Nephrol. 9, 881–888 (2014).
Perico, N. et al. Sirolimus therapy to halt the progression of ADPKD. J. Am. Soc. Nephrol. 21, 1031–1040 (2010).
Ruggenenti, P. et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 68, 206–216 (2005).
Kim, Y. et al. Automated segmentation of kidneys from MR images in patients with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 11, 576–584 (2016).
Spithoven, E. M. et al. Estimation of total kidney volume in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 66, 792–801 (2015).
Perrone, R. D. et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int.Rep. 2, 442–450 (2017).
Casteleijn, N. F. et al. Urine and plasma osmolality in patients with autosomal dominant polycystic kidney disease: reliable indicators of vasopressin activity and disease prognosis? Am. J. Nephrol. 41, 248–256 (2015).
Torres, V. E., Bankir, L. & Grantham, J. J. A case for water in the treatment of polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 4, 1140–1150 (2009).
Zittema, D. et al. Kidney function and plasma copeptin levels in healthy kidney donors and autosomal dominant polycystic kidney disease patients. Clin. J. Am. Soc. Nephrol. 9, 1553–1562 (2014).
Boertien, W. E. et al. Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: results from the CRISP cohort. Am. J. Kidney Dis. 61, 420–429 (2013).
Boertien, W. E. et al. Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 27, 4131–4137 (2012).
Corradi, V. et al. Copeptin levels and kidney function in ADPKD: case-control study. Clin. Nephrol. 86, 147–153 (2016).
Meijer, E. et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 361–368 (2011).
Nakajima, A., Lu, Y., Kawano, H., Horie, S. & Muto, S. Association of arginine vasopressin surrogate marker urinary copeptin with severity of autosomal dominant polycystic kidney disease (ADPKD). Clin. Exp. Nephrol. 19, 1199–1205 (2015).
Pejchinovski, M. et al. Urine peptidome analysis predicts risk of end-stage renal disease and reveals proteolytic pathways involved in autosomal dominant polycystic kidney disease progression. Nephrol. Dial. Transplant. 32, 487–497 (2017).
Salih, M. et al. Proteomics of urinary vesicles links plakins and complement to polycystic kidney disease. J. Am. Soc. Nephrol. 27, 3079–3092 (2016).
Street, J. M., Koritzinsky, E. H., Glispie, D. M. & Yuen, P. S. T. Urine exosome isolation and characterization. Methods Mol. Biol. 1641, 413–423 (2017).
Hogan, M. C. et al. Identification of biomarkers for PKD1 using urinary exosomes. J. Am. Soc. Nephrol. 26, 1661–1670 (2015).
Kistler, A. D. et al. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS ONE 8, e53016 (2013).
Chapman, A. B., Stepniakowski, K. & Rahbari-Oskoui, F. Hypertension in autosomal dominant polycystic kidney disease. Adv. Chron. Kidney Dis. 17, 153–163 (2010).
Chapman, A. B. & Gabow, P. A. Hypertension in autosomal dominant polycystic kidney disease. Kidney Int. Suppl. 61, S71–S73 (1997).
Johnson, A. M. & Gabow, P. A. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J. Am. Soc. Nephrol. 8, 1560–1567 (1997).
Yu, A. S. et al. Trajectory of the GFR in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, abstr., FR-OR006 (2016).
Schrier, R. W. Hypertension and autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 57, 811–813 (2011).
Ecder, T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Curr. Hypertens. Rev. 9, 2–11 (2013).
Chapman, A. B. et al. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 8, 1292–1297 (1997).
Chapman, A. B., Johnson, A., Gabow, P. A. & Schrier, R. W. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N. Engl. J. Med. 323, 1091–1096 (1990).
Kip, S. N. et al. [Ca2+]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: relevance to the ADPKD phenotype. Circ. Res. 96, 873–880 (2005).
Rahbari-Oskoui, F., Williams, O. & Chapman, A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 29, 2194–2201 (2014).
Hessheimer, A. J., Vidal, O., Valentini, M. & Garcia-Valdecasas, J. C. Pheochromocytoma as a rare cause of arterial hypertension in a patient with autosomal dominant polycystic kidney disease: a diagnostic and therapeutic dilemma. Int. J. Surg. Case Rep. 14, 85–88 (2015).
Hoorn, E. J. et al. A case of primary aldosteronism revealed after renal transplantation. Nat. Rev. Nephrol. 7, 55–60 (2011).
Schrier, R. W. ACE inhibitors, left ventricular mass and renal cyst growth in ADPKD. Pharmacol Res. 114, 166–168 (2016).
Nutahara, K. et al. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin. Pract. 99, c18–c23 (2005).
Zeltner, R., Poliak, R., Stiasny, B., Schmieder, R. E. & Schulze, B. D. Renal and cardiac effects of antihypertensive treatment with ramipril versus metoprolol in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 23, 573–579 (2008).
van Dijk, M. A., Breuning, M. H., Duiser, R., van Es, L. A. & Westendorp, R. G. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 18, 2314–2320 (2003).
Ecder, T. et al. Diuretics versus angiotensin-converting enzyme inhibitors in autosomal dominant polycystic kidney disease. Am. J. Nephrol. 21, 98–103 (2001).
Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
Klahr, S. et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. J. Am. Soc. Nephrol. 5, 2037–2047 (1995).
Appel, L. J. et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N. Engl. J. Med. 363, 918–929 (2010).
Ku, E. et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int. 87, 1055–1060 (2015).
Group, S. R. et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 373, 2103–2116 (2015).
Ruzicka, M., Burns, K. D. & Hiremath, S. Precision medicine for hypertension management in chronic kidney disease: relevance of SPRINT for therapeutic targets in nondiabetic renal disease. Can. J. Cardiol. 33, 611–618 (2017).
Gansevoort, R. T. et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 Trial. Nephrol. Dial.Transplant. 31, 1887–1894 (2016).
Akinbodewa, A. A., Adejumo, O. A., Ogunsemoyin, A. O., Osasan, S. A. & Adefolalu, O. A. Co-existing autosomal dominant polycystic kidney disease and nephrotic syndrome in a Nigerian patient with lupus nephritis. Ann. Afr. Med. 15, 83–86 (2016).
Yenigun, E. C. et al. Coexistence of autosomal dominant polycystic kidney disease and amyloidosis in a patient with nephrotic-range proteinuria. Iran. J. Kidney Dis. 8, 243–245 (2014).
Torres, V. E. et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 640–647 (2011).
Torres, V. E. et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 81, 577–585 (2012).
Torres, V. E. et al. Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: results from the TEMPO 3:4 Trial. Clin. J. Am. Soc. Nephrol. 11, 803–811 (2016).
Devuyst, O. et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 Trial. J. Am. Soc. Nephrol. 28, 1592–1602 (2017).
Casteleijn, N. F. et al. Tolvaptan and kidney pain in patients with autosomal dominant polycystic kidney disease: secondary analysis from a randomized controlled trial. Am. J. Kidney Dis. 69, 210–219 (2017).
Torres, V. E. et al. Rationale and design of a clinical trial investigating tolvaptan safety and efficacy in autosomal dominant polycystic kidney disease. Am. J. Nephrol. 45, 257–266 (2017).
Zand, L. et al. Renal hemodynamic effects of the HMG-CoA reductase inhibitors in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 31, 1290–1295 (2016).
Namli, S. et al. Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail 29, 55–59 (2007).
van Dijk, M. A., Kamper, A. M., van Veen, S., Souverijn, J. H. & Blauw, G. J. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 16, 2152–2157 (2001).
Fassett, R. G., Coombes, J. S., Packham, D., Fairley, K. F. & Kincaid-Smith, P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scand. J. Urol. Nephrol. 44, 56–61 (2010).
Brosnahan, G. et al. Effect of statin therapy on the progression of autosomal dominant polycystic kidney disease. A secondary analysis of the HALT PKD trials. Curr. Hypertens. Rev. http://dx.doi.org/10.2174/1573402113666170427142815 (2017).
Myint, T. M., Rangan, G. K. & Webster, A. C. Treatments to slow progression of autosomal dominant polycystic kidney disease: systematic review and meta-analysis of randomized trials. Nephrology 19, 217–226 (2014).
van Keimpema, L. et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 137, 1661–1668.e2 (2009).
Hogan, M. C. et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol. Dial. Transplant. 27, 3532–3539 (2012).
Meijer, E. et al. Rationale and design of the DIPAK 1 study: a randomized controlled clinical trial assessing the efficacy of lanreotide to Halt disease progression in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 63, 446–455 (2014).
Lantinga, M. A. et al. Hepatic cyst infection during use of the somatostatin analog lanreotide in autosomal dominant polycystic kidney disease: an interim analysis of the randomized open-label multicenter DIPAK-1 study. Drug Saf. 40, 153–167 (2017).
Novalic, Z. et al. Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J. Am. Soc. Nephrol. 23, 842–853 (2012).
Hajarnis, S. et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 8, 14395 (2017).
Yheskel, M. & Patel, V. Therapeutic microRNAs in polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 26, 282–289 (2017).
Knight, T. et al. Medical resource utilization and costs associated with autosomal dominant polycystic kidney disease in the USA: a retrospective matched cohort analysis of private insurer data. Clinicoecon. Outcomes Res. 7, 123–132 (2015).
Neuville, M., Hustinx, R., Jacques, J., Krzesinski, J. M. & Jouret, F. Diagnostic algorithm in the management of acute febrile abdomen in patients with autosomal dominant polycystic kidney disease. PLoS ONE 11, e0161277 (2016).
Hogan, M. C. & Norby, S. M. Evaluation and management of pain in autosomal dominant polycystic kidney disease. Adv. Chron. Kidney Dis. 17, e1–e16 (2010).
Chapman, A. B. et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 88, 17–27 (2015).
Suwabe, T. et al. Clinical features of cyst infection and hemorrhage in ADPKD: new diagnostic criteria. Clin. Exp. Nephrol. 16, 892–902 (2012).
Chapman, A. B., Gabow, P. A. & Schrier, R. W. Reversible renal failure associated with angiotensin-converting enzyme inhibitors in polycystic kidney disease. Ann. Intern. Med. 115, 769–773 (1991).
Lantinga, M. A. et al. International multi-specialty delphi survey: identification of diagnostic criteria for hepatic and renal cyst infection. Nephron 134, 205–214 (2016).
Lantinga, M. A., Drenth, J. P. & Gevers, T. J. Diagnostic criteria in renal and hepatic cyst infection. Nephrol. Dial. Transplant. 30, 744–751 (2015).
Lantinga, M. A., de Sevaux, R. G. & Drenth, J. P. 18F-FDG PET/CT during diagnosis and follow-up of recurrent hepatic cyst infection in autosomal dominant polycystic kidney disease. Clin. Nephrol. 84, 61–64 (2015).
Suwabe, T. et al. Intracystic magnetic resonance imaging in patients with autosomal dominant polycystic kidney disease: features of severe cyst infection in a case-control study. BMC Nephrol. 17, 170 (2016).
Piccoli, G. B. et al. Positron emission tomography in the diagnostic pathway for intracystic infection in adpkd and “cystic” kidneys. a case series. BMC Nephrol. 12, 48 (2011).
Lantinga, M. A. et al. Management of renal cyst infection in patients with autosomal dominant polycystic kidney disease: a systematic review. Nephrol. Dial. Transplant. 32, 144–150 (2017).
Suwabe, T. et al. Cyst infection in autosomal dominant polycystic kidney disease: causative microorganisms and susceptibility to lipid-soluble antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 34, 1369–1379 (2015).
Nishiura, J. L. et al. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin. J. Am. Soc. Nephrol. 4, 838–844 (2009).
Torres, V. E., Wilson, D. M., Hattery, R. R. & Segura, J. W. Renal stone disease in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 22, 513–519 (1993).
Grampsas, S. A. et al. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 36, 53–57 (2000).
Umbreit, E. C. et al. Percutaneous nephrolithotomy for large or multiple upper tract calculi and autosomal dominant polycystic kidney disease. J. Urol. 183, 183–187 (2010).
Mallett, A., Patel, M., Tunnicliffe, D. J. & Rangan, G. K. KHA-CARI autosomal dominant polycystic kidney disease guideline: management of renal stone disease. Semin. Nephrol. 35, 603–606.e3 (2015).
Penniston, K. L., Wertheim, M. L., Nakada, S. Y. & Jhagroo, R. A. Factors associated with patient recall of individualized dietary recommendations for kidney stone prevention. Eur. J. Clin. Nutr. 70, 1062–1067 (2016).
Fink, H. A. et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann. Intern. Med. 158, 535–543 (2013).
Casteleijn, N. F. et al. Novel treatment protocol for ameliorating refractory, chronic pain in patients with autosomal dominant polycystic kidney disease. Kidney Int. 91, 972–981 (2017).
Miskulin, D. C. et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1-4: a cross-sectional study. Am. J. Kidney Dis. 63, 214–226 (2014).
Casteleijn, N. F. et al. A stepwise approach for effective management of chronic pain in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. 29 (Suppl. 4), iv142–iv153 (2014).
World Health Organisation. Cancer pain relief: with a guide to opiod availability — 2nd ed. (WHO, 1996).
Ballantyne, J. C., Kalso, E. & Stannard, C. WHO analgesic ladder: a good concept gone astray. BMJ 352, i20 (2016).
Tellman, M. W., Bahler, C. D., Shumate, A. M., Bacallao, R. L. & Sundaram, C. P. Management of pain in autosomal dominant polycystic kidney disease and anatomy of renal innervation. J. Urol. 193, 1470–1478 (2015).
PKD Foundation forum. Living with PKD: pain relief and medical marijuana. PKD Foundation http://forums.pkdconnection.org/viewtopic.php?f=3&t=109 (2017).
Walsh, N. & Sarria, J. E. Management of chronic pain in a patient with autosomal dominant polycystic kidney disease by sequential celiac plexus blockade, radiofrequency ablation, and spinal cord stimulation. Am. J. Kidney Dis. 59, 858–861 (2012).
Loukas, M. et al. A review of the thoracic splanchnic nerves and celiac ganglia. Clin. Anat. 23, 512–522 (2010).
Casteleijn, N. F., de Jager, R. L., Neeleman, M. P., Blankestijn, P. J. & Gansevoort, R. T. Chronic kidney pain in autosomal dominant polycystic kidney disease: a case report of successful treatment by catheter-based renal denervation. Am. J. Kidney Dis. 63, 1019–1021 (2014).
Valente, J. F., Dreyer, D. R., Breda, M. A. & Bennett, W. M. Laparoscopic renal denervation for intractable ADPKD-related pain. Nephrol. Dial. Transplant. 16, 160 (2001).
Chapuis, O., Sockeel, P., Pallas, G., Pons, F. & Jancovici, R. Thoracoscopic renal denervation for intractable autosomal dominant polycystic kidney disease-related pain. Am. J. Kidney Dis. 43, 161–163 (2004).
Bhatt, D. L. et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 370, 1393–1401 (2014).
Haseebuddin, M. et al. Long-term impact of laparoscopic cyst decortication on renal function, hypertension and pain control in patients with autosomal dominant polycystic kidney disease. J. Urol. 188, 1239–1244 (2012).
Millar, M. et al. Surgical cyst decortication in autosomal dominant polycystic kidney disease. J. Endourol. 27, 528–534 (2013).
Cook, N. R. et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334, 885–888 (2007).
Torres, V. E. et al. Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney Int. 91, 493–500 (2017).
McMahon, E. J., Campbell, K. L., Bauer, J. D. & Mudge, D. W. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev., CD010070 (2015).
Dougher, C. E. et al. Spot urine sodium measurements do not accurately estimate dietary sodium intake in chronic kidney disease. Am. J. Clin. Nutr. 104, 298–305 (2016).
Thomas, M. C. et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 34, 861–866 (2011).
Mente, A., O'Donnell, M. J. & Yusuf, S. Sodium and cardiovascular disease — Authors' reply. Lancet 388, 2113–2114 (2016).
O'Donnell, M. et al. Dietary sodium and cardiovascular disease risk. N. Engl. J. Med. 375, 2404–2406 (2016).
O'Donnell, M., Mente, A. & Yusuf, S. Commentary: Accepting what we don't know will lead to progress. Int. J. Epidemiol. 45, 260–262 (2016).
Campbell, N. R. Dissidents & dietary sodium: concerns about the commentary by O'Donnell et al. Int. J. Epidemiol. 46, 362–366 (2017).
Graudal, N., Jurgens, G., Baslund, B. & Alderman, M. H. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am. J. Hypertens. 27, 1129–1137 (2014).
Vegter, S. et al. Sodium intake, ACE inhibition, and progression to ESRD. J. Am. Soc. Nephrol. 23, 165–173 (2012).
Lambers Heerspink, H. J. et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 82, 330–337 (2012).
Maroni, B. J., Steinman, T. I. & Mitch, W. E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 27, 58–65 (1985).
Ko, G. J., Obi, Y., Tortorici, A. R. & Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 20, 77–85 (2017).
Kalantar-Zadeh, K. et al. North American experience with low protein diet for non-dialysis-dependent chronic kidney disease. BMC Nephrol. 17, 90 (2016).
Ma, Y. et al. A dietary quality comparison of popular weight-loss plans. J. Am. Diet Assoc. 107, 1786–1791 (2007).
Levey, A. S. et al. Effect of dietary protein restriction on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 48, 879–888 (2006).
Fouque, D. & Laville, M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev, CD001892 (2009).
Boertien, W. E. et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am. J. Kidney Dis. 65, 833–841 (2015).
Wang, C. J., Grantham, J. J. & Wetmore, J. B. The medicinal use of water in renal disease. Kidney Int. 84, 45–53 (2013).
Barash, I., Ponda, M. P., Goldfarb, D. S. & Skolnik, E. Y. A pilot clinical study to evaluate changes in urine osmolality and urine cAMP in response to acute and chronic water loading in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 5, 693–697 (2010).
Wang, C. J., Creed, C., Winklhofer, F. T. & Grantham, J. J. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin. J. Am. Soc. Nephrol. 6, 192–197 (2011).
Higashihara, E. et al. Does increased water intake prevent disease progression in autosomal dominant polycystic kidney disease? Nephrol. Dial. Transplant. 29, 1710–1719 (2014).
Lacquaniti, A. et al. Apelin and copeptin: two opposite biomarkers associated with kidney function decline and cyst growth in autosomal dominant polycystic kidney disease. Peptides 49, 1–8 (2013).
Kocer, D., Karakukcu, C., Ozturk, F., Eroglu, E. & Kocyigit, I. Evaluation of fibrosis markers: apelin and transforming growth factor-β1 in autosomal dominant polycystic kidney disease patients. Ther. Apher. Dial. 20, 517–522 (2016).
Helal, I. et al. Serum uric acid, kidney volume and progression in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. 28, 380–385 (2013).
Kocyigit, I. et al. Serum uric acid levels and endothelial dysfunction in patients with autosomal dominant polycystic kidney disease. Nephron Clin. Pract. 123, 157–164 (2013).
Akiyama, K., Mochizuki, T., Kataoka, H., Tsuchiya, K. & Nitta, K. Fibroblast growth factor 23 and soluble Klotho in patients with autosomal dominant polycystic kidney disease. Nephrology http://dx.doi.org/10.1111/nep.12862 (2016).
Pavik, I. et al. Soluble klotho and autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 7, 248–257 (2012).
Pavik, I. et al. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol. Dial. Transplant. 28, 352–359 (2013).
Meijer, E. et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am. J. Kidney Dis. 56, 883–895 (2010).
Parikh, C. R. et al. Evaluation of urine biomarkers of kidney injury in polycystic kidney disease. Kidney Int. 81, 784–790 (2012).
Harskamp, L. R. et al. Urinary EGF receptor ligand excretion in patients with autosomal dominant polycystic kidney disease and response to tolvaptan. Clin. J. Am. Soc. Nephrol. 10, 1749–1756 (2015).
Zschiedrich, S. et al. Secreted frizzled-related protein 4 predicts progression of autosomal dominant polycystic kidney disease. Nephrol. Dial Transplant 31, 284–289 (2016).
Ruggenenti, P. et al. Effect of sirolimus on disease progression in patients with autosomal dominant polycystic kidney disease and CKD stages 3b-4. Clin. J. Am. Soc. Nephrol. 11, 785–794 (2016).
Acknowledgements
M.B.L. is supported by the American Society of Nephrology Jared J. Grantham fellowship.
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
A.B.C. declares an association with Otsuka Pharmaceutical Group through membership of the TEMPO steering committee. M.B.L. declares no competing interests.
Related links
Rights and permissions
About this article
Cite this article
Lanktree, M., Chapman, A. New treatment paradigms for ADPKD: moving towards precision medicine. Nat Rev Nephrol 13, 750–768 (2017). https://doi.org/10.1038/nrneph.2017.127
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.127
This article is cited by
-
Autosomal dominant polycystic kidney disease in Colombia
BMC Nephrology (2023)
-
Network medicine: an approach to complex kidney disease phenotypes
Nature Reviews Nephrology (2023)
-
Ganoderic acid A is the effective ingredient of Ganoderma triterpenes in retarding renal cyst development in polycystic kidney disease
Acta Pharmacologica Sinica (2020)
-
Two cases of fungal cyst infection in ADPKD: is this really a rare complication?
BMC Infectious Diseases (2019)
-
A single-arm pilot study of metformin in patients with autosomal dominant polycystic kidney disease
BMC Nephrology (2019)