Key Points
-
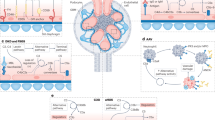
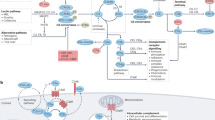
The complement system is a critical modulator of immune responses that triages microbial and other threats through pattern recognition, tailored effector functions, and intensive crosstalk with other systems
-
When disrupted by dysregulation or age-related effects, or excessively triggered by acute and chronic tissue damage, biomaterials or transplants, complement can attack host cells and contribute to inflammatory conditions
-
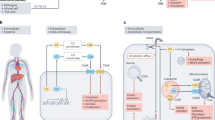
The kidney is particularly sensitive to complement-mediated damage, exemplified by the involvement of complement in several kidney diseases (such as atypical haemolytic uraemic syndrome (aHUS) and C3 glomerulopathies) and in complications of kidney transplantation and haemodialysis
-
Therapeutic complement inhibition is successfully used in paroxysmal nocturnal haemoglobinuria and aHUS, and has shown promise in other clinical conditions, raising hope for improved treatment options for other disorders
Abstract
Although the complement system is primarily perceived as a host defence system, a more versatile, yet potentially more harmful side of this innate immune pathway as an inflammatory mediator also exists. The activities that define the ability of the complement system to control microbial threats and eliminate cellular debris — such as sensing molecular danger patterns, generating immediate effectors, and extensively coordinating with other defence pathways — can quickly turn complement from a defence system to an aggressor that drives immune and inflammatory diseases. These host-offensive actions become more pronounced with age and are exacerbated by a variety of genetic factors and autoimmune responses. Complement can also be activated inappropriately, for example in response to biomaterials or transplants. A wealth of research over the past two decades has led to an increasingly finely tuned understanding of complement activation, identified tipping points between physiological and pathological behaviour, and revealed avenues for therapeutic intervention. This Review summarizes our current view of the key activating, regulatory, and effector mechanisms of the complement system, highlighting important crosstalk connections, and, with an emphasis on kidney disease and transplantation, discusses the involvement of complement in clinical conditions and promising therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Ricklin, D. & Lambris, J. D. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 190, 3831–3838 (2013).
Reis, E. S. et al. Applying complement therapeutics to rare diseases. Clin. Immunol. 161, 225–240 (2015).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part I — molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015).
Merle, N. S., Noe, R., Halbwachs-Mecarelli, L., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part II: role in immunity. Front. Immunol. 6, 257 (2015).
Mastellos, D. C., Deangelis, R. A. & Lambris, J. D. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 25, 29–38 (2013).
Stephan, A. H., Barres, B. A. & Stevens, B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 35, 369–389 (2012).
Kohl, J. The role of complement in danger sensing and transmission. Immunol. Res. 34, 157–176 (2006).
Scott, D. & Botto, M. The paradoxical roles of C1q and C3 in autoimmunity. Immunobiology http://dx.doi.org/10.1016/j.imbio.2015.05.001 (2015).
Kolev, M., Le Friec, G. & Kemper, C. Complement — tapping into new sites and effector systems. Nat. Rev. Immunol. 14, 811–820 (2014).
Harris, C. L., Heurich, M., Rodriguez de Cordoba, S. & Morgan, B. P. The complotype: dictating risk for inflammation and infection. Trends Immunol. 33, 513–521 (2012).
Diebolder, C. A. et al. Complement is activated by IgG hexamers assembled at the cell surface. Science 343, 1260–1263 (2014).
Kouser, L. et al. Emerging and novel functions of complement protein C1q. Front. Immunol. 6, 317 (2015).
Harboe, M. et al. The down-stream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Mol. Immunol. 47, 373–380 (2009).
Harboe, M., Ulvund, G., Vien, L., Fung, M. & Mollnes, T. E. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 138, 439–446 (2004).
Nilsson, B. & Nilsson Ekdahl, K. The tick-over theory revisited: is C3 a contact-activated protein? Immunobiology 217, 1106–1110 (2012).
Morgan, B. P. The membrane attack complex as an inflammatory trigger. Immunobiology http://dx.doi.org/10.1016/j.imbio.2015.04.006 (2015).
Taylor, R. P. & Lindorfer, M. A. The role of complement in mAb-based therapies of cancer. Methods 65, 18–27 (2014).
Klos, A., Wende, E., Wareham, K. J. & Monk, P. N. International Union of Basic and Clinical Pharmacology. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol. Rev. 65, 500–543 (2013); erratum 66, 466 (2014)
Coulthard, L. G. & Woodruff, T. M. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J. Immunol. 194, 3542–3548 (2015).
Nordahl, E. A. et al. Activation of the complement system generates antibacterial peptides. Proc. Natl Acad. Sci. USA 101, 16879–16884 (2004).
Ghebrehiwet, B., Hosszu, K. K., Valentino, A., Ji, Y. & Peerschke, E. I. Monocyte expressed macromolecular C1 and C1q receptors as molecular sensors of danger: implications in SLE. Front. Immunol. 5, 278 (2014).
Helmy, K. Y. et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124, 915–927 (2006).
Zeerleder, S. C1-inhibitor: more than a serine protease inhibitor. Semin. Thromb. Hemost 37, 362–374 (2011).
Matsushita, M., Endo, Y. & Fujita, T. Structural and functional overview of the lectin complement pathway: its molecular basis and physiological implication. Arch. Immunol. Ther. Exp. (Warsz) 61, 273–283 (2013).
Clark, S. J. & Bishop, P. N. Role of factor H and related proteins in regulating complement activation in the macula, and relevance to age-related macular degeneration. J. Clin. Med. 4, 18–31 (2015).
Reis, E. S. et al. C5a receptor-dependent cell activation by physiological concentrations of desarginated C5a: insights from a novel label-free cellular assay. J. Immunol. 189, 4797–4805 (2012).
Lesher, A. M., Nilsson, B. & Song, W. C. Properdin in complement activation and tissue injury. Mol. Immunol. 56, 191–198 (2013).
Kemper, C., Atkinson, J. P. & Hourcade, D. E. Properdin: emerging roles of a pattern-recognition molecule. Annu. Rev. Immunol. 28, 131–155 (2010).
Marsh, J. E., Zhou, W. & Sacks, S. H. Local tissue complement synthesis — fine tuning a blunt instrument. Arch. Immunol. Ther. Exp. (Warsz.) 49, S41–S46 (2001).
Conway, E. M. Reincarnation of ancient links between coagulation and complement. J. Thromb. Haemost. 13, S121–S132 (2015).
Ritis, K. et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 177, 4794–4802 (2006).
Rayes, J. et al. The interaction between factor H and VWF increases factor H cofactor activity and regulates VWF prothrombotic status. Blood 123, 121–125 (2014).
Speth, C. et al. Complement and platelets: mutual interference in the immune network. Mol. Immunol. 67, 108–118 (2015).
Hamad, O. A. et al. Contact activation of C3 enables tethering between activated platelets and polymorphonuclear leukocytes via CD11b/CD18. Thromb. Haemost. 114, 1207–1217 (2015).
Ali, H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol. Lett. 128, 36–45 (2010).
Bohlson, S. S., O'Conner, S. D., Hulsebus, H. J., Ho, M. M. & Fraser, D. A. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front. Immunol. 5, 402 (2014).
Camous, L. et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 117, 1340–1349 (2011).
Wang, H., Wang, C., Zhao, M. H. & Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 181, 518–527 (2015).
Wu, M. C. et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc. Natl Acad. Sci. USA 110, 9439–9444 (2013).
Hsieh, C. C. et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood 121, 1760–1768 (2013).
Markiewski, M. M. et al. Modulation of the antitumor immune response by complement. Nat. Immunol. 9, 1225–1235 (2008).
Son, M., Santiago-Schwarz, F., Al-Abed, Y. & Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl Acad. Sci. USA 109, E3160–E3167 (2012).
Li, K. et al. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology 217, 65–73 (2012).
Hawlisch, H. et al. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity 22, 415–426 (2005).
Kemper, C. & Kohl, J. Novel roles for complement receptors in T cell regulation and beyond. Mol. Immunol. 56, 181–190 (2013).
Dunkelberger, J., Zhou, L., Miwa, T. & Song, W. C. C5aR expression in a novel GFP reporter gene knockin mouse: implications for the mechanism of action of C5aR signaling in T cell immunity. J. Immunol. 188, 4032–4042 (2012).
Karsten, C. M. et al. Monitoring and cell-specific deletion of C5aR1 using a novel floxed GFP–C5aR1 reporter knock-in mouse. J. Immunol. 194, 1841–1855 (2015).
Cardone, J. et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat. Immunol. 11, 862–871 (2010).
Le Friec, G. et al. The CD46–Jagged1 interaction is critical for human TH1 immunity. Nat. Immunol. 13, 1213–1221 (2012).
Ghannam, A., Fauquert, J. L., Thomas, C., Kemper, C. & Drouet, C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol. Immunol. 58, 98–107 (2014).
Liszewski, M. K. et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39, 1143–1157 (2013).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353 (2015).
Hajishengallis, G. & Lambris, J. D. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 31, 154–163 (2010).
Morgan, B. P. & Gasque, P. Extrahepatic complement biosynthesis: where, when and why? Clin. Exp. Immunol. 107, 1–7 (1997).
Leemans, J. C., Kors, L., Anders, H. J. & Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 10, 398–414 (2014).
Cheung, K. P., Kasimsetty, S. G. & McKay, D. B. Innate immunity in donor procurement. Curr. Opin. Organ Transplant. 18, 154–160 (2013).
McCullough, J. W., Renner, B. & Thurman, J. M. The role of the complement system in acute kidney injury. Semin. Nephrol. 33, 543–556 (2013).
Noris, M. & Remuzzi, G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and C3 glomerulopathy: core curriculum 2015. Am. J. Kidney Dis. 66, 359–375 (2015).
Sacks, S. H. & Zhou, W. The role of complement in the early immune response to transplantation. Nat. Rev. Immunol. 12, 431–442 (2012).
Song, W. C. Crosstalk between complement and toll-like receptors. Toxicol. Pathol. 40, 174–182 (2012).
Bosmann, M. et al. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J. Immunol. 188, 5086–5093 (2012).
Seow, V. et al. Inflammatory responses induced by lipopolysaccharide are amplified in primary human monocytes but suppressed in macrophages by complement protein C5a. J. Immunol. 191, 4308–4316 (2013).
Ling, G. S. et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat. Commun. 5, 3039 (2014).
Zou, L. et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J. Immunol. 191, 5625–5635 (2013).
Triantafilou, M., Hughes, T. R., Morgan, B. P. & Triantafilou, K. Complementing the inflammasome. Immunology 147, 152–164 (2016).
Asgari, E. et al. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 122, 3473–3481 (2013).
Laudisi, F. et al. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1β release. J. Immunol. 191, 1006–1010 (2013).
Triantafilou, K., Hughes, T. R., Triantafilou, M. & Morgan, B. P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell Sci. 126, 2903–2913 (2013).
Samstad, E. O. et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J. Immunol. 192, 2837–2845 (2014).
Benoit, M. E., Clarke, E. V., Morgado, P., Fraser, D. A. & Tenner, A. J. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J. Immunol. 188, 5682–5693 (2012).
Pinto, M. R., Melillo, D., Giacomelli, S., Sfyroera, G. & Lambris, J. D. Ancient origin of the complement system: emerging invertebrate models. Adv. Exp. Med. Biol. 598, 372–388 (2007).
Flajnik, M. F. Re-evaluation of the immunological Big Bang. Curr. Biol. 24, R1060–R1065 (2014).
Sunyer, J. O. et al. Evolution of complement as an effector system in innate and adaptive immunity. Immunol. Res. 27, 549–564 (2003).
Carroll, M. C. & Isenman, D. E. Regulation of humoral immunity by complement. Immunity 37, 199–207 (2012).
Dempsey, P. W., Allison, M. E., Akkaraju, S., Goodnow, C. C. & Fearon, D. T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271, 348–350 (1996).
Humphrey, J. H., Grennan, D. & Sundaram, V. The origin of follicular dendritic cells in the mouse and the mechanism of trapping of immune complexes on them. Eur. J. Immunol. 14, 859–864 (1984).
Karsten, C. M. & Kohl, J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology 217, 1067–1079 (2012).
Huang, Z. Y. et al. Interaction of two phagocytic host defense systems: Fcγ receptors and complement receptor 3. J. Biol. Chem. 286, 160–168 (2011).
Karsten, C. M. et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 18, 1401–1406 (2012).
Bosmann, M. & Ward, P. A. The inflammatory response in sepsis. Trends Immunol. 34, 129–136 (2013).
Ward, P. A. The dark side of C5a in sepsis. Nat. Rev. Immunol. 4, 133–142 (2004).
Silasi-Mansat, R. et al. Complement inhibition decreases early fibrogenic events in the lung of septic baboons. J. Cell. Mol. Med. 19, 2549–2563 (2015).
Silasi-Mansat, R. et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood 116, 1002–1010 (2010).
Barratt-Due, A. et al. Combined inhibition of complement C5 and CD14 markedly attenuates inflammation, thrombogenicity, and hemodynamic changes in porcine sepsis. J. Immunol. 191, 819–827 (2013).
Egge, K. H. et al. The anti-inflammatory effect of combined complement and CD14 inhibition is preserved during escalating bacterial load. Clin. Exp. Immunol. 181, 457–467 (2015).
Burk, A. M. et al. Early complementopathy after multiple injuries in humans. Shock 37, 348–354 (2012).
Ganter, M. T. et al. Role of the alternative pathway in the early complement activation following major trauma. Shock 28, 29–34 (2007).
Huber-Lang, M., Kovtun, A. & Ignatius, A. The role of complement in trauma and fracture healing. Semin. Immunol. 25, 73–78 (2013).
Rafail, S. et al. Complement deficiency promotes cutaneous wound healing in mice. J. Immunol. 194, 1285–1291 (2015).
Brennan, F. H. et al. The complement receptor C5aR controls acute inflammation and astrogliosis following spinal cord injury. J. Neurosci. 35, 6517–6531 (2015).
Chen, M., Daha, M. R. & Kallenberg, C. G. The complement system in systemic autoimmune disease. J. Autoimmun. 34, J276–286 (2010).
Chen, M. & Kallenberg, C. G. ANCA-associated vasculitides — advances in pathogenesis and treatment. Nat. Rev. Rheumatol. 6, 653–664 (2010).
Banz, Y. & Rieben, R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann. Med. 44, 205–217 (2012).
Zipfel, P. F. et al. The role of complement in C3 glomerulopathy. Mol. Immunol. 67, 21–30 (2015).
Orsini, F., De Blasio, D., Zangari, R., Zanier, E. R. & De Simoni, M. G. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front. Cell. Neurosci. 8, 380 (2014).
Veerhuis, R., Nielsen, H. M. & Tenner, A. J. Complement in the brain. Mol. Immunol. 48, 1592–1603 (2011).
Hovland, A. et al. The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis. Atherosclerosis 241, 480–494 (2015).
Morgan, B. P. The role of complement in neurological and neuropsychiatric diseases. Expert Rev. Clin. Immunol. 11, 1109–1119 (2015).
Patzelt, J., Verschoor, A. & Langer, H. F. Platelets and the complement cascade in atherosclerosis. Front. Physiol. 6, 49 (2015).
McHarg, S., Clark, S. J., Day, A. J. & Bishop, P. N. Age-related macular degeneration and the role of the complement system. Mol. Immunol. 67, 43–50 (2015).
Ekdahl, K. N. et al. Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 63, 1042–1050 (2011).
Grumach, A. S. & Kirschfink, M. Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol. Immunol. 61, 110–117 (2014).
Risitano, A. M. Paroxysmal nocturnal hemoglobinuria and other complement-mediated hematological disorders. Immunobiology 217, 1080–1087 (2012).
Rodriguez, E., Rallapalli, P. M., Osborne, A. J. & Perkins, S. J. New functional and structural insights from updated mutational databases for complement factor H, Factor I, membrane cofactor protein and C3. Biosci. Rep. 34, 5 (2014).
Hageman, G. S. et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl Acad. Sci. USA 102, 7227–7232 (2005).
Weismann, D. et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478, 76–81 (2011).
Heurich, M. et al. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl Acad. Sci. USA 108, 8761–8766 (2011).
Kurts, C., Panzer, U., Anders, H. J. & Rees, A. J. The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Song, D., Zhou, W., Sheerin, S. H. & Sacks, S. H. Compartmental localization of complement component transcripts in the normal human kidney. Nephron 78, 15–22 (1998).
Noris, M. & Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 33, 479–492 (2013).
Clark, S. J. et al. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J. Immunol. 190, 2049–2057 (2013).
Jozsi, M., Tortajada, A., Uzonyi, B., Goicoechea de Jorge, E. & Rodriguez de Cordoba, S. Factor H-related proteins determine complement-activating surfaces. Trends Immunol. 36, 374–384 (2015).
Noris, M., Mescia, F. & Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8, 622–633 (2012).
Kenawy, H. I., Boral, I. & Bevington, A. Complement-coagulation cross-talk: a potential mediator of the physiological activation of complement by low pH. Front. Immunol. 6, 215 (2015).
George, J. N. & Nester, C. M. Syndromes of thrombotic microangiopathy. N. Engl. J. Med. 371, 654–666 (2014).
Riedl, M. et al. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin. Thromb. Hemost 40, 444–464 (2014).
Mele, C., Remuzzi, G. & Noris, M. Hemolytic uremic syndrome. Semin. Immunopathol. 36, 399–420 (2014).
Noris, M. et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood 124, 1715–1726 (2014).
Frimat, M. et al. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 122, 282–292 (2013).
Zuber, J. et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat. Rev. Nephrol. 8, 643–657 (2012).
Cofiell, R. et al. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 125, 3253–3262 (2015).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2015).
Meri, S. Complement activation in diseases presenting with thrombotic microangiopathy. Eur. J. Intern. Med. 24, 496–502 (2013).
Morigi, M. et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J. Immunol. 187, 172–180 (2011).
Nester, C. M. & Brophy, P. D. Eculizumab in the treatment of atypical haemolytic uraemic syndrome and other complement-mediated renal diseases. Curr. Opin. Pediatr. 25, 225–231 (2013).
Pecoraro, C. et al. Treatment of congenital thrombotic thrombocytopenic purpura with eculizumab. Am. J. Kidney Dis. 66, 1067–1070 (2015).
Phillips, E. H. et al. The role of ADAMTS-13 activity and complement mutational analysis in differentiating acute thrombotic microangiopathies. J. Thromb. Haemost. 14, 175–185 (2016).
Tsai, E., Chapin, J., Laurence, J. C. & Tsai, H. M. Use of eculizumab in the treatment of a case of refractory, ADAMTS13-deficient thrombotic thrombocytopenic purpura: additional data and clinical follow-up. Br. J. Haematol. 162, 558–559 (2013).
Pickering, M. C. et al. C3 glomerulopathy: consensus report. Kidney Int. 84, 1079–1089 (2013).
Cook, H. T. & Pickering, M. C. Histopathology of MPGN and C3 glomerulopathies. Nat. Rev. Nephrol. 11, 14–22 (2015).
Servais, A. et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 82, 454–464 (2012).
Martinez-Barricarte, R. et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J. Clin. Invest. 120, 3702–3712 (2010).
Xiao, X., Pickering, M. C. & Smith, R. J. C3 glomerulopathy: the genetic and clinical findings in dense deposit disease and C3 glomerulonephritis. Semin. Thromb. Hemost. 40, 465–471 (2014).
Damman, J. et al. Hypoxia and complement-and-coagulation pathways in the deceased organ donor as the major target for intervention to improve renal allograft outcome. Transplantation 99, 1293–1300 (2015).
Amura, C. R. et al. Complement activation and toll-like receptor-2 signaling contribute to cytokine production after renal ischemia/reperfusion. Mol. Immunol. 52, 249–257 (2012).
Farrar, C. A. et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J. Clin. Invest. 126, 1911–19 (2016).
Muller, T. F., Kraus, M., Neumann, C. & Lange, H. Detection of renal allograft rejection by complement components C5A and TCC in plasma and urine. J. Lab. Clin. Med. 129, 62–71 (1997).
Stites, E., Le Quintrec, M. & Thurman, J. M. The complement system and antibody-mediated transplant rejection. J. Immunol. 195, 5525–5531 (2015).
Pratt, J. R., Basheer, S. A. & Sacks, S. H. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat. Med. 8, 582–587 (2002).
Strainic, M. G., Shevach, E. M., An, F., Lin, F. & Medof, M. E. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat. Immunol. 14, 162–171 (2013).
van der Touw, W. et al. Cutting edge: receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J. Immunol. 190, 5921–5925 (2013).
Marsh, J. E. et al. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation 72, 1310–1318 (2001).
Thomas, K. A., Valenzuela, N. M. & Reed, E. F. The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection. Trends Mol. Med. 21, 319–329 (2015).
Chen Song, S. et al. Complement inhibition enables renal allograft accommodation and long-term engraftment in presensitized nonhuman primates. Am. J. Transplant. 11, 2057–2066 (2011).
Fremeaux-Bacchi, V. & Legendre, C. M. The emerging role of complement inhibitors in transplantation. Kidney Int. 88, 967–973 (2015).
DeAngelis, R. A., Reis, E. S., Ricklin, D. & Lambris, J. D. Targeted complement inhibition as a promising strategy for preventing inflammatory complications in hemodialysis. Immunobiology 217, 1097–1105 (2012).
Kourtzelis, I. et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood 116, 631–639 (2010).
Ricklin, D. & Lambris, J. D. Complement in immune and inflammatory disorders: therapeutic interventions. J. Immunol. 190, 3839–3847 (2013).
Licht, C. et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 87, 1061–1073 (2015).
Wong, E. K. & Kavanagh, D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl. Res. 165, 306–320 (2015).
Eskandary, F., Wahrmann, M., Muhlbacher, J. & Bohmig, G. A. Complement inhibition as potential new therapy for antibody-mediated rejection. Transpl. Int. 29, 392–402 (2016).
Stegall, M. D. et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am. J. Transplant. 11, 2405–2413 (2011).
Cornell, L. D., Schinstock, C. A., Gandhi, M. J., Kremers, W. K. & Stegall, M. D. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am. J. Transplant. 15, 1293–2302 (2015).
Ricklin, D. & Lambris, J. D. Therapeutic control of complement activation at the level of the central component C3. Immunobiology http://dx.doi.org/10.1016/j.imbio.2015.06.012 (2015).
Zhang, Y. et al. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J. Am. Soc. Nephrol. 24, 1820–1829 (2013).
Sacks, S. et al. Targeting complement at the time of transplantation. Adv. Exp. Med. Biol. 735, 247–255 (2013).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02247531 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02247479 (2016).
Zhang, Y. et al. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology 220, 993–998 (2015).
Risitano, A. M. et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood 123, 2094–2101 (2014).
Reis, E. S. et al. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology 220, 476–482 (2015).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02264639 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02503332 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02588833 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02502903 (2015).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02222545 (2015).
Vo, A. A. et al. A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients. Transplantation 99, 299–308 (2015).
ChemoCentryx. ChemoCentryx announces positive results in phase II ANCA-associated vasculitis CLEAR trial of orally administered complement 5a receptor inhibitor CCX168. http://ir.chemocentryx.com/releasedetail.cfm?releaseid=948998 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02384317 (2016).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02464891 (2015).
US National Library of Science. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02246595 (2016).
Hillmen, P. et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 162, 62–73 (2013).
Wu, M. A., Zanichelli, A., Mansi, M. & Cicardi, M. Current treatment options for hereditary angioedema due to C1 inhibitor deficiency. Expert Opin. Pharmacother. 17, 27–40 (2016).
Banda, N. K. et al. Mechanisms of mannose-binding lectin-associated serine proteases-1/3 activation of the alternative pathway of complement. Mol. Immunol. 49, 281–289 (2011).
Dobo, J. et al. Multiple roles of complement MASP-1 at the interface of innate immune response and coagulation. Mol. Immunol. 61, 69–78 (2014).
Arnold, J. N., Royle, L., Dwek, R. A., Rudd, P. M. & Sim, R. B. Human immunoglobulin glycosylation and the lectin pathway of complement activation. Adv. Exp. Med. Biol. 564, 27–43 (2005).
Amara, U. et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 185, 5628–5636 (2010).
Inforzato, A. et al. PTX3 as a paradigm for the interaction of pentraxins with the complement system. Semin. Immunol. 25, 79–85 (2013).
Csincsi, A. I. et al. Factor H-related protein 5 interacts with pentraxin 3 and the extracellular matrix and modulates complement activation. J. Immunol. 194, 4963–4973 (2015).
Trouw, L. A., Blom, A. M. & Gasque, P. Role of complement and complement regulators in the removal of apoptotic cells. Mol. Immunol. 45, 1199–1207 (2008).
Acknowledgements
The authors thank Deborah McClellan for editing the manuscript before submission. J.D.L. also thanks Ralph and Sallie Weaver for the generous endowment of his professorship. Given the broad scope of this Review, we often refer to specialized review articles rather than primary literature, and we have only been able to include selected examples of the breadth of transformative work in the field; we therefore thank all our colleagues who are not specifically cited for their great contributions and their understanding. This work was supported by grants from the US NIH (AI068730, AI030040, DE021685) and the National Science Foundation (grant No. 1423304) and by funding from the European Community's Seventh Framework Programme, under grant agreement number 602699 (DIREKT).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussion of the article's content, writing, and review/editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
J.D.L and D.R. are inventors of patents or patent applications that describe the use of complement inhibitors for therapeutic purposes. J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors. E.S.R. declares no competing interests.
Related links
Glossary
- Convertase
-
A protein complex transiently formed by an opsonin (C3b, C4b) and a protease fragment (Bb, C2b), which activates complement components C3 or C5 and promotes opsonization, amplification and/or generation of effector molecules.
- Anaphylatoxin
-
A widely used term for complement effector fragments C3a and C5a, which are released upon activation of C3 and C5, respectively, and exert numerous immunomodulatory, chemotactic and/or cell-activating functions.
- Opsonization
-
The process of depositing complement proteins on a particle surface to facilitate its removal. In the complement system, C3b, C4b (and their degradation fragments) and C1q facilitate phagocytosis via their receptors on immune cells.
- Amplification
-
A major function of the 'alternative pathway' of complement, whereby convertase-mediated deposition of C3b on a targeted surface enables the formation of additional C3 convertases and deposition of more C3b. In absence of regulators, these events lead to rapid opsonization of the attacked cell or particle.
- Consumption
-
A result of continuous and poorly controlled activation of complement components in the circulation, leading to a depletion of the native plasma protein and affecting the responsiveness of the cascade.
- Fluid phase
-
Refers to complement activity in the circulation rather than on the targeted surface. Fluid-phase activation maintains alertness of the complement system (tick-over) but needs to be tightly controlled by soluble complement regulators.
Rights and permissions
About this article
Cite this article
Ricklin, D., Reis, E. & Lambris, J. Complement in disease: a defence system turning offensive. Nat Rev Nephrol 12, 383–401 (2016). https://doi.org/10.1038/nrneph.2016.70
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.70
This article is cited by
-
Cross-sectional and Longitudinal Associations Between Metal Mixtures and Serum C3, C4: Result from the Manganese‑exposed Workers Healthy Cohort
Biological Trace Element Research (2024)
-
Androgen-regulated stromal complement component 7 (C7) suppresses prostate cancer growth
Oncogene (2023)
-
Complement component C1q is an immunological rheostat that regulates Fc:Fc\(\gamma\)R interactions
Immunogenetics (2023)
-
Persistently elevated complement alternative pathway biomarkers in COVID-19 correlate with hypoxemia and predict in-hospital mortality
Medical Microbiology and Immunology (2022)
-
The complement C3-complement factor D-C3a receptor signalling axis regulates cardiac remodelling in right ventricular failure
Nature Communications (2022)