Key Points
-
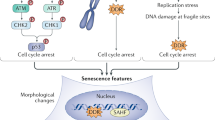
Cellular senescence is a multi-faceted programme involved in diverse physiological and pathological processes including embryonic development, regeneration and repair, cancer-protection, ageing, and disease
-
Senescent cells that are transiently present (acute senescence) are beneficial, whereas prolonged signalling and aberrant accumulation of senescent cells (chronic senescence) impairs renal function and promotes kidney disease
-
Chronic senescent cells accumulate in the kidneys during natural ageing and have been causally linked to age-related decline in renal function
-
Senescent cell accumulation also occurs in association with several renal diseases and therapeutic damage, and correlates with disease progression or deterioration in several instances
-
Therapeutic interventions that target senescent cells, termed senotherapies, have potential to attenuate age-related renal dysfunction, improve disease outcome, and ensure success of kidney transplantation
-
Development of effective and safe senotherapies should greatly benefit from future research aimed at understanding of the location, origin and properties of senescent cells in greater detail
Abstract
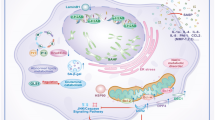
The senescence programme is implicated in diverse biological processes, including embryogenesis, tissue regeneration and repair, tumorigenesis, and ageing. Although in vivo studies of senescence are in their infancy, evidence suggesting that senescent cells are a heterogeneous cell type is accumulating: senescence can be induced by different stressors, and senescent cells have varying degrees of genomic and epigenomic instability and different cell origins, contributing to their diversity. Two main classes of senescent cells have been identified: acute and chronic senescent cells. Acute senescent cells are generated during coordinated, beneficial biological processes characterized by a defined senescence trigger, transient senescent-cell signalling functions, and eventual senescent-cell clearance. In contrast, chronic senescent cells arise more slowly from cumulative, diverse stresses and are inefficiently eliminated, leading to their accumulation and deleterious effects through a secretory phenotype. Senescent cells have been identified in many tissues and organs, including the kidney. Here, we discuss the emerging roles of senescent cells in renal development, homeostasis, and pathology. We also address how senotherapy, or targeting of senescent cells, might be used to improve renal function with normal ageing, disease, or therapy-induced damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Flatt, T. A new definition of aging? Front. Genet. 3, 148 (2012).
Rose, M. R. Evolutionary Biology of Aging (Oxford Univ. Press, 1991).
Williams, G. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Baker, D. J. et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 10, 825–836 (2008).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Taddei, M. L. et al. Senescent stroma promotes prostate cancer progression: the role of miR-210. Mol. Oncol. 8, 1729–1746 (2014).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Iannello, A., Thompson, T. W., Ardolino, M., Lowe, S. W. & Raulet, D. H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 210, 2057–2069 (2013).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Bayreuther, K. et al. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc. Natl Acad. Sci. USA 85, 5112–5116 (1988).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Zhang, H., Xiong, Y. & Beach, D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell 4, 897–906 (1993).
Rodier, F. et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979 (2009).
Jun, J. I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 (2010).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Kim, S. H. et al. Upregulation of chicken p15INK4b at senescence and in the developing brain. J. Cell Sci. 119, 2435–2443 (2006).
Fuchs, Y. & Steller, H. Programmed cell death in animal development and disease. Cell 147, 742–758 (2011).
Zhu, F. et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS ONE 8, e74535 (2013).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Munoz-Espin, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Sagiv, A. et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 8, 328–344 (2016).
Wolstein, J. M. et al. INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol. 299, F1486–F1495 (2010).
Baisantry, A. et al. Autophagy induces prosenescent changes in proximal tubular S3 segments. J. Am. Soc. Nephrol. 27, 1609–1616 (2016).
Megyesi, J. et al. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 60, 2164–2172 (2001).
Young, A. R. et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 (2009).
Kang, C. et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349, aaa5612 (2015).
Liu, S. et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8, 826–837 (2012).
Kimura, T. et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 22, 902–913 (2011).
Braun, H. et al. Cellular senescence limits regenerative capacity and allograft survival. J. Am. Soc. Nephrol. 23, 1467–1473 (2012).
Collado, M. & Serrano, M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer 6, 472–476 (2006).
Sharpless, N. E., Ramsey, M. R., Balasubramanian, P., Castrillon, D. H. & DePinho, R. A. The differential impact of p16INK4a or p19ARF deficiency on cell growth and tumorigenesis. Oncogene 23, 379–385 (2004).
Cole, A. M. et al. p21 loss blocks senescence following Apc loss and provokes tumourigenesis in the renal but not the intestinal epithelium. EMBO Mol. Med. 2, 472–486 (2010).
Young, A. P. et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat. Cell Biol. 10, 361–369 (2008).
Capparelli, C. et al. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle 11, 2285–2302 (2012).
Farmaki, E. et al. Selection of p53-deficient stromal cells in the tumor microenvironment. Genes Cancer 3, 592–598 (2012).
Yang, G. et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl Acad. Sci. USA 103, 16472–16477 (2006).
Burd, C. E. et al. Monitoring tumorigenesis and senescence in vivo with a p16INK4a-luciferase model. Cell 152, 340–351 (2013).
Sansoni, P. et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood 82, 2767–2773 (1993).
Min, H., Montecino-Rodriguez, E. & Dorshkind, K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J. Immunol. 176, 1007–1012 (2006).
Chung, H. Y. et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. 8, 18–30 (2009).
Bernet, J. D. et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 20, 265–271 (2014).
Cosgrove, B. D. et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014).
Garcia-Prat, L. et al. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42 (2016).
Chen, R. et al. Telomerase deficiency causes alveolar stem cell senescence-associated low-grade inflammation in lungs. J. Biol. Chem. 290, 30813–30829 (2015).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Sone, H. & Kagawa, Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48, 58–67 (2005).
Zhou, Z. et al. Accelerated senescence of endothelial progenitor cells in hypertension is related to the reduction of calcitonin gene-related peptide. J. Hypertens. 28, 931–939 (2010).
Imanishi, T., Moriwaki, C., Hano, T. & Nishio, I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J. Hypertens. 23, 1831–1837 (2005).
Westhoff, J. H. et al. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 52, 123–129 (2008).
Joosten, S. A. et al. Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am. J. Pathol. 162, 1305–1312 (2003).
Melk, A., Schmidt, B. M., Vongwiwatana, A., Rayner, D. C. & Halloran, P. F. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am. J. Transplant. 5, 1375–1382 (2005).
Ablain, J. et al. Activation of a promyelocytic leukemia-tumor protein 53 axis underlies acute promyelocytic leukemia cure. Nat. Med. 20, 167–174 (2014).
Dorr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013).
Le, O. N. et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 9, 398–409 (2010).
Lee, M. O. et al. Effect of ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscler. Thromb. Vasc. Biol. 32, 343–352 (2012).
Darmady, E. M., Offer, J. & Woodhouse, M. A. The parameters of the ageing kidney. J. Pathol. 109, 195–207 (1973).
Tan, J. C. et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 78, 686–692 (2010).
Rule, A. D. et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann. Intern. Med. 152, 561–567 (2010).
Elsherbiny, H. E. et al. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin. J. Am. Soc. Nephrol. 9, 1892–1902 (2014).
Rule, A. D. et al. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am. J. Kidney Dis. 59, 611–618 (2012).
Wang, X. et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 85, 677–685 (2014).
Lorenz, E. C. et al. Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol. Dial. Transplant. 26, 2695–2700 (2011).
Rule, A. D. & Glassock, R. J. The Aging Kidney (UpToDate, 2016).
Esposito, C. & Dal Canton, A. Functional changes in the aging kidney. J. Nephrol. 23 (Suppl. 15), S41–S45 (2010).
Lindeman, R. D., Tobin, J. & Shock, N. W. Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc. 33, 278–285 (1985).
Choudhury, D. & Levi, M. Kidney aging — inevitable or preventable? Nat. Rev. Nephrol. 7, 706–717 (2011).
Schmitt, R. & Melk, A. New insights on molecular mechanisms of renal aging. Am. J. Transplant. 12, 2892–2900 (2012).
Clements, M. E., Chaber, C. J., Ledbetter, S. R. & Zuk, A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE 8, e70464 (2013).
Berkenkamp, B. et al. In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS ONE 9, e88071 (2014).
Yang, H. C. & Fogo, A. B. Fibrosis and renal aging. Kidney Int. Suppl. 4, 75–78 (2014).
McGlynn, L. M. et al. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell 8, 45–51 (2009).
Naesens, M. Replicative senescence in kidney aging, renal disease, and renal transplantation. Discov. Med. 11, 65–75 (2011).
Tullius, S. G. et al. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann. Surg. 252, 662–674 (2010).
Schmitt, R., Susnik, N. & Melk, A. Molecular aspects of renal senescence. Curr. Opin. Organ. Transplant. 20, 412–416 (2015).
Slegtenhorst, B. R. et al. Mechanisms and consequences of injury and repair in older organ transplants. Transplantation 97, 1091–1099 (2014).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004).
Chkhotua, A. B. et al. Increased expression of p16INK4a and p27Kip1 cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am. J. Kidney Dis. 41, 1303–1313 (2003).
Melk, A. et al. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 65, 510–520 (2004).
Ding, G. et al. Tubular cell senescence and expression of TGF-beta1 and p21WAF1/CIP1 in tubulointerstitial fibrosis of aging rats. Exp. Mol. Pathol. 70, 43–53 (2001).
Melk, A. et al. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 63, 2134–2143 (2003).
Sis, B. et al. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int. 71, 218–226 (2007).
Liu, J. et al. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl Res. 159, 454–463 (2012).
Verzola, D. et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 295, F1563–F1573 (2008).
Koppelstaetter, C. et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell 7, 491–497 (2008).
Melk, A. et al. Effects of donor age and cell senescence on kidney allograft survival. Am. J. Transplant. 9, 114–123 (2009).
Vinuesa, E. et al. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J. Pathol. 214, 104–113 (2008).
Xue, J. L. et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. 17, 1135–1142 (2006).
Ferenbach, D. A. & Bonventre, J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 11, 264–276 (2015).
Rahman, M., Shad, F. & Smith, M. C. Acute kidney injury: a guide to diagnosis and management. Am. Fam. Physician 86, 631–639 (2012).
Canaud, G. & Bonventre, J. V. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transplant. 30, 575–583 (2015).
Xu, X. et al. Aging aggravates long-term renal ischemia-reperfusion injury in a rat model. J. Surg. Res. 187, 289–296 (2014).
Tumlin, J. A., Madaio, M. P. & Hennigar, R. Idiopathic IgA nephropathy: pathogenesis, histopathology, and therapeutic options. Clin. J. Am. Soc. Nephrol. 2, 1054–1061 (2007).
Lu, Y. Y. et al. Proteins induced by telomere dysfunction are associated with human IgA nephropathy. J. Zhejiang Univ. Sci. B 15, 566–574 (2014).
Kalyani, R. R. & Egan, J. M. Diabetes and altered glucose metabolism with aging. Endocrinol. Metab. Clin. North Am. 42, 333–347 (2013).
Minamino, T. et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15, 1082–1087 (2009).
Markowski, D. N. et al. HMGA2 expression in white adipose tissue linking cellular senescence with diabetes. Genes Nutr. 8, 449–456 (2013).
Cao, Z. & Cooper, M. E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2, 243–247 (2011).
Mora-Fernandez, C. et al. Diabetic kidney disease: from physiology to therapeutics. J. Physiol. 592, 3997–4012 (2014).
Kitada, K. et al. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J. Diabetes Complications 28, 604–611 (2014).
Wolf, G., Reinking, R., Zahner, G., Stahl, R. A. & Shankland, S. J. Erk 1,2 phosphorylates p27Kip1: functional evidence for a role in high glucose-induced hypertrophy of mesangial cells. Diabetologia 46, 1090–1099 (2003).
Wolf, G., Schroeder, R., Zahner, G., Stahl, R. A. & Shankland, S. J. High glucose-induced hypertrophy of mesangial cells requires p27Kip1, an inhibitor of cyclin-dependent kinases. Am. J. Pathol. 158, 1091–1100 (2001).
Zhang, X. et al. Downregulation of connexin 43 expression by high glucose induces senescence in glomerular mesangial cells. J. Am. Soc. Nephrol. 17, 1532–1542 (2006).
Al-Douahji, M. et al. The cyclin kinase inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in experimental diabetic nephropathy. Kidney Int. 56, 1691–1699 (1999).
Wolf, G., Schanze, A., Stahl, R. A., Shankland, S. J. & Amann, K. p27Kip1 knockout mice are protected from diabetic nephropathy: evidence for p27Kip1 haplotype insufficiency. Kidney Int. 68, 1583–1589 (2005).
Morocutti, A. et al. Premature senescence of skin fibroblasts from insulin-dependent diabetic patients with kidney disease. Kidney Int. 50, 250–256 (1996).
Torres, V. E., Harris, P. C. & Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 (2007).
Hildebrandt, F., Benzing, T. & Katsanis, N. Ciliopathies. N. Engl. J. Med. 364, 1533–1543 (2011).
Nadasdy, T. et al. Proliferative activity of cyst epithelium in human renal cystic diseases. J. Am. Soc. Nephrol. 5, 1462–1468 (1995).
Igarashi, P. & Somlo, S. Genetics and pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 13, 2384–2398 (2002).
Park, J. Y. et al. p21 is decreased in polycystic kidney disease and leads to increased epithelial cell cycle progression: roscovitine augments p21 levels. BMC Nephrol. 8, 12 (2007).
Bukanov, N. O., Smith, L. A., Klinger, K. W., Ledbetter, S. R. & Ibraghimov-Beskrovnaya, O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444, 949–952 (2006).
Park, J. Y., Park, S. H. & Weiss, R. H. Disparate effects of roscovitine on renal tubular epithelial cell apoptosis and senescence: implications for autosomal dominant polycystic kidney disease. Am. J. Nephrol. 29, 509–515 (2009).
Hildebrandt, F., Attanasio, M. & Otto, E. Nephronophthisis: disease mechanisms of a ciliopathy. J. Am. Soc. Nephrol. 20, 23–35 (2009).
Lu, D. et al. Loss of Glis2/NPHP7 causes kidney epithelial cell senescence and suppresses cyst growth in the Kif3a mouse model of cystic kidney disease. Kidney Int. 89, 1307–1323 (2016).
Kremers, W. K. et al. Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: the Aging Kidney Anatomy study. Nephrol. Dial. Transplant. 30, 2034–2039 (2015).
Kooman, J. P., van der Sande, F. M. & Leunissen, K. M. Kidney disease and aging: a reciprocal relation. Exp. Gerontol. http://dx.doi.org/10.1016/j.exger.2016.02.003 (2016).
Kooman, J. P., Kotanko, P., Schols, A. M., Shiels, P. G. & Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10, 732–742 (2014).
Jia, T. et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 14, 116 (2013).
Quimby, J. M. et al. Feline chronic kidney disease is associated with shortened telomeres and increased cellular senescence. Am. J. Physiol. Renal Physiol. 305, F295–F303 (2013).
Klinkhammer, B. M. et al. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS ONE 9, e92115 (2014).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
Megyesi, J., Safirstein, R. L. & Price, P. M. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J. Clin. Invest. 101, 777–782 (1998).
Zhou, H. et al. The induction of cell cycle regulatory and DNA repair proteins in cisplatin-induced acute renal failure. Toxicol. Appl. Pharmacol. 200, 111–120 (2004).
Wen, J. et al. Aging increases the susceptibility of cisplatin-induced nephrotoxicity. Age (Dordr.) 37, 112 (2015).
Childs, B., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Kamada, H. et al. Synthesis of a poly(vinylpyrrolidone-co-dimethyl maleic anhydride) co-polymer and its application for renal drug targeting. Nat. Biotechnol. 21, 399–404 (2003).
Franssen, E. J., Moolenaar, F., de Zeeuw, D. & Meijer, D. K. Low-molecular-weight proteins as carriers for renal drug targeting. Contrib. Nephrol. 101, 99–103 (1993).
Lin, Y. et al. Targeted drug delivery to renal proximal tubule epithelial cells mediated by 2-glucosamine. J. Control Release 167, 148–156 (2013).
Wischnjow, A. et al. Renal targeting: peptide-based drug delivery to proximal tubule cells. Bioconjug. Chem. 27, 1050–1057 (2016).
Wen, Z. Z. et al. Angiotensin II receptor blocker attenuates intrarenal renin-angiotensin-system and podocyte injury in rats with myocardial infarction. PLoS ONE 8, e67242 (2013).
Kunieda, T. et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114, 953–960 (2006).
Fan, Y. Y. et al. Aldosterone/mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology 152, 680–688 (2011).
Choi, C. H., Zuckerman, J. E., Webster, P. & Davis, M. E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl Acad. Sci. USA 108, 6656–6661 (2011).
Kamaly, N., He, J. C., Ausiello, D. A. & Farokhzad, O. C. Nanomedicines for renal disease: current status and future applications. Nat. Rev. Nephrol. 12, 738–753 (2016).
Tuffin, G., Waelti, E., Huwyler, J., Hammer, C. & Marti, H. P. Immunoliposome targeting to mesangial cells: a promising strategy for specific drug delivery to the kidney. J. Am. Soc. Nephrol. 16, 3295–3305 (2005).
Molitoris, B. A. et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 20, 1754–1764 (2009).
Xu, X. M. et al. Anti-inflamm-aging effects of long-term caloric restriction via overexpression of SIGIRR to inhibit NF-kappaB signaling pathway. Cell. Physiol. Biochem. 37, 1257–1270 (2015).
Heydari, A. R., Unnikrishnan, A., Lucente, L. V. & Richardson, A. Caloric restriction and genomic stability. Nucleic Acids Res. 35, 7485–7496 (2007).
Ning, Y. C. et al. Short-term calorie restriction protects against renal senescence of aged rats by increasing autophagic activity and reducing oxidative damage. Mech. Ageing Dev. 134, 570–579 (2013).
Inoki, K., Kim, J. & Guan, K. L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 52, 381–400 (2012).
Iglesias-Bartolome, R. et al. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 11, 401–414 (2012).
Zhuo, L. et al. Expression and mechanism of mammalian target of rapamycin in age-related renal cell senescence and organ aging. Mech. Ageing Dev. 130, 700–708 (2009).
Kawai, M., Kinoshita, S., Ozono, K. & Michigami, T. Inorganic phosphate activates the AKT/mTORC1 pathway and shortens the life span of an alpha-klotho-deficient model. J. Am. Soc. Nephrol. 27, 2810–2824 (2016).
Noren Hooten, N. et al. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 15, 572–581 (2016).
Piwkowska, A. et al. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem. Biophys. Res. Commun. 393, 268–273 (2010).
Lee, M. J. et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal Physiol. 292, F617–F627 (2007).
Morales, A. I. et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 77, 861–869 (2010).
Li, J. et al. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKalpha-regulated autophagy induction. Sci. Rep. 6, 23975 (2016).
Hoenicke, L. & Zender, L. Immune surveillance of senescent cells — biological significance in cancer- and non-cancer pathologies. Carcinogenesis 33, 1123–1126 (2012).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Yosef, R. et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 (2016).
Russo, M., Spagnuolo, C., Tedesco, I., Bilotto, S. & Russo, G. L. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem. Pharmacol. 83, 6–15 (2012).
O'Hare, T. et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 65, 4500–4505 (2005).
Zhu, Y. et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Iwasa, H., Han, J. & Ishikawa, F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells 8, 131–144 (2003).
Chien, Y. et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136 (2011).
Alimbetov, D. et al. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology 17, 305–315 (2016).
Laberge, R. M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Moiseeva, O. et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 12, 489–498 (2013).
Tasdemir, N. et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 6, 612–629 (2016).
Ewald, J. A., Desotelle, J. A., Wilding, G. & Jarrard, D. F. Therapy-induced senescence in cancer. J. Natl Cancer Inst. 102, 1536–1546 (2010).
Ramakrishna, G. et al. Role of cellular senescence in hepatic wound healing and carcinogenesis. Eur. J. Cell Biol. 91, 739–747 (2012).
Kim, K. H., Chen, C. C., Monzon, R. I. & Lau, L. F. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol. Cell. Biol. 33, 2078–2090 (2013).
O'Leary, B., Finn, R. S. & Turner, N. C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 13, 417–430 (2016).
Barroso-Sousa, R., Shapiro, G. I. & Tolaney, S. M. Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care (Basel) 11, 167–173 (2016).
DiRocco, D. P. et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Renal Physiol. 306, F379–F388 (2014).
Kurz, D. J., Decary, S., Hong, Y. & Erusalimsky, J. D. Senescence-associated β-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 113, 3613–3622 (2000).
Georgakopoulou, E. A. et al. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5, 37–50 (2013).
d'Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522 (2008).
Aird, K. M. & Zhang, R. Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol. Biol. 965, 185–196 (2013).
Kopp, H. G., Hooper, A. T., Shmelkov, S. V. & Rafii, S. Beta-galactosidase staining on bone marrow. The osteoclast pitfall. Histol. Histopathol. 22, 971–976 (2007).
Holt, D. J. & Grainger, D. W. Senescence and quiescence induced compromised function in cultured macrophages. Biomaterials 33, 7497–7507 (2012).
Yang, N. C. & Hu, M. L. The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp. Gerontol. 40, 813–819 (2005).
Traves, P. G., Lopez-Fontal, R., Luque, A. & Hortelano, S. The tumor suppressor ARF regulates innate immune responses in mice. J. Immunol. 187, 6527–6538 (2011).
Shapiro, G. I. et al. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 55, 505–509 (1995).
Ohtani, N., Yamakoshi, K., Takahashi, A. & Hara, E. Real-time in vivo imaging of p16gene expression: a new approach to study senescence stress signaling in living animals. Cell Div. 5, 1 (2010).
el-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K. & Elledge, S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816 (1993).
Rayess, H., Wang, M. B. & Srivatsan, E. S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 130, 1715–1725 (2012).
Ben-Porath, I. & Weinberg, R. A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37, 961–976 (2005).
Hochegger, K. et al. p21 and mTERT are novel markers for determining different ischemic time periods in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 292, F762–F768 (2007).
Author information
Authors and Affiliations
Contributions
All authors researched the data, discussed the article's content, wrote the text and reviewed or edited the article before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Senescence-associated secretory phenotype
-
(SASP). High amounts of pro-inflammatory and matrix-degrading molecules produced and secreted by senescent cells.
- Immune surveillance
-
Mechanism by which senescent cells are detected and eliminated by the immune system.
- Mesonephros
-
Transitory embryonic excretory organ derived from the metanephric mesenchyme that forms around embryonic day (E) 9 and degenerates by E15.5 in mice.
- Functional reserve capacity
-
Capacity an organ to preserve function, if damage should occur.
- Ciliopathies
-
Group of diseases that arise from mutations in genes encoding primary cilia-related proteins and that affect several organs such as the eyes, limbs and kidneys.
Rights and permissions
About this article
Cite this article
Sturmlechner, I., Durik, M., Sieben, C. et al. Cellular senescence in renal ageing and disease. Nat Rev Nephrol 13, 77–89 (2017). https://doi.org/10.1038/nrneph.2016.183
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.183
This article is cited by
-
Uremic toxins mediate kidney diseases: the role of aryl hydrocarbon receptor
Cellular & Molecular Biology Letters (2024)
-
Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Inhibition of ACSS2-mediated histone crotonylation alleviates kidney fibrosis via IL-1β-dependent macrophage activation and tubular cell senescence
Nature Communications (2024)
-
Cellular senescence of renal tubular epithelial cells in acute kidney injury
Cell Death Discovery (2024)
-
Yearning for machine learning: applications for the classification and characterisation of senescence
Cell and Tissue Research (2023)