Key Points
-
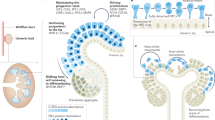
The basic architecture of the kidney is established during early embryonic development through iterative branching of the ureteric bud
-
Epithelial–mesenchymal cell interactions at the termini of the branching epithelium determine when and how many nephrons are generated
-
Genetic and/or environmental perturbations during kidney development can alter the developmental processes that direct branching and nephron formation and could, hence, underlie congenital and acquired nephron deficits
-
Nephron number in humans is determined at fetal stages and is emerging as a critical factor that influences adult health
Abstract
The mammalian kidney develops from a simple epithelial bud to an arborized network of tubules, which are fated to form the ureter, renal pelvis and collecting ducts. This process of ductal elaboration is achieved through an ancient developmental mechanism known as branching morphogenesis that is widely employed in glandular organs, the vasculature and lungs. It breaks up large solid tissues facilitating secretion, excretion and gas exchange, depending on the tissue. In the kidney, growth of the ureteric bud is driven by interactions between progenitor cells in the tips of the epithelial tree and their mesenchymal 'caps'. The cells of the cap mesenchyme give rise to nephrons; therefore, the interaction between these two cell populations is likely to be a critical driver of nephron number, which is determined during gestation. These cellular interactions are potentially affected by genetic mutations (congenital kidney diseases) and by changes in the fetal environment. Understanding the aetiology of congenital and acquired kidney diseases therefore requires a full appreciation of the processes involved in establishing the cellular architecture of the kidney and of the factors that affect the commitment of progenitor cells to form nephrons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Romagnani, P., Lasagni, L. & Remuzzi, G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat. Rev. Nephrol. 9, 137–146 (2013).
Hoy, W. E. et al. The early development of the kidney and implications for future health. J. Dev. Orig. Health Dis. 1, 216–233 (2010).
Bertram, J. F., Douglas-Denton, R. N., Diouf, B., Hughson, M. D. & Hoy, W. E. Human nephron number: implications for health and disease. Pediatr. Nephrol. 26, 1529–1533 (2011).
Hughson, M. D., Douglas-Denton, R., Bertram, J. F. & Hoy, W. E. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 69, 671–678 (2006).
Keller, G., Zimmer, G., Mall, G., Ritz, E. & Amann, K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 348, 101–108 (2003).
Luyckx, V. A. et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382, 273–283 (2013).
White, S. L. et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am. J. Kidney Dis. 54, 248–261 (2009).
Fraser, E. A. The development of the vertebrate excretory system. Biol. Rev. Camb. Philos. Soc. 25, 159–187 (1950).
Obara-Ishihara, T., Kuhlman, J., Niswander, L. & Herzlinger, D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126, 1103–1108 (1999).
Hall, R. W. The development of the mesonephros and the Mullerian ducts in amphibia. Bull. Museum Comparative Zool. 45, 31–125 (1904).
de Martino, C. & Zamboni, L. A morphologic study of the mesonephros of the human embryo. J. Ultrastruct. Res. 16, 399–427 (1966).
Kupffer, C. Untersunchungen uber die Entwicklung des Harn- und Geschlechtsystems. I. Arch. Mikroskop. Anat. Entwicklungmech. 1, 233–248 (in German) (1865).
Osathanondh, V. & Potter, E. L. Development of human kidney as shown by microdissection. III. Formation and interrelationship of collecting tubules and nephrons. Arch. Pathol. 76, 290–302 (1963).
Saxen, L. & Sariola, H. Early organogenesis of the kidney. Pediatr. Nephrol. 1, 385–392 (1987).
Short, K. M. et al. Global quantification of tissue dynamics in the developing mouse kidney. Dev. Cell 29, 188–202 (2014).
Oliver, J. Nephrons and Kidneys: a Quantitative Study of Developmental and Evolutionary Mammalian Renal Archtectonics (Harper & Row, 1968).
Brunskill, E. W. et al. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev. Cell 15, 781–791 (2008).
Georgas, K. et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev. Biol. 332, 273–286 (2009).
Kao, R. M., Vasilyev, A., Miyawaki, A., Drummond, I. A. & McMahon, A. P. Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J. Am. Soc. Nephrol. 23, 1682–1690 (2012).
Self, M. et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 25, 5214–5228 (2006).
Kreidberg, J. A. et al. WT-1 is required for early kidney development. Cell 74, 679–691 (1993).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Xu, J. et al. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev. Cell 31, 434–447 (2014).
Bouchard, M., Souabni, A., Mandler, M., Neubuser, A. & Busslinger, M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 16, 2958–2970 (2002).
Grote, D. et al. Gata3 acts downstream of β-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet. 4, e1000316 (2008).
Grote, D., Souabni, A., Busslinger, M. & Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133, 53–61 (2006).
Marose, T. D., Merkel, C. E., McMahon, A. P. & Carroll, T. J. β-Catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev. Biol. 314, 112–126 (2008).
Tsang, T. E. et al. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev. Biol. 223, 77–90 (2000).
Pedersen, A., Skjong, C. & Shawlot, W. Lim1 is required for nephric duct extension and ureteric bud morphogenesis. Dev. Biol. 288, 571–581 (2005).
Wellik, D. M., Hawkes, P. J. & Capecchi, M. R. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 16, 1423–1432 (2002).
Brophy, P. D., Ostrom, L., Lang, K. M. & Dressler, G. R. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128, 4747–4756 (2001).
Torres, M., Gomez-Pardo, E., Dressler, G. R. & Gruss, P. Pax-2 controls multiple steps of urogenital development. Development 121, 4057–4065 (1995).
Xu, P. X. et al. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 23, 113–117 (1999).
Esquela, A. F. & Lee, S. J. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev. Biol. 257, 356–370 (2003).
James, R. G., Kamei, C. N., Wang, Q., Jiang, R. & Schultheiss, T. M. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133, 2995–3004 (2006).
Nishinakamura, R. et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128, 3105–3115 (2001).
Kume, T., Deng, K. & Hogan, B. L. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127, 1387–1395 (2000).
Grieshammer, U. et al. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev. Cell 6, 709–717 (2004).
Wainwright, E. N., Wilhelm, D., Combes, A. N., Little, M. H. & Koopman, P. ROBO2 restricts the nephrogenic field and regulates Wolffian duct–nephrogenic cord separation. Dev. Biol. 404, 88–102 (2015).
Michos, O. et al. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134, 2397–2405 (2007).
Enomoto, H. et al. GFRα1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 21, 317–324 (1998).
Sanchez, M. P. et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382, 70–73 (1996).
Schuchardt, A., D'Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994).
Bridgewater, D. et al. Canonical WNT/β-catenin signaling is required for ureteric branching. Dev. Biol. 317, 83–94 (2008).
Chi, X. et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev. Cell 17, 199–209 (2009).
Mugford, J. W., Sipila, P., McMahon, J. A. & McMahon, A. P. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1- dependent nephron progenitor compartment within the mammalian kidney. Dev. Biol. 324, 88–98 (2008).
Costantini, F. in Kidney Development, Disease, Repair and Regeneration (ed. Little, M.) 41–53 (Academic Press, 2016).
Fisher, C. E., Michael, L., Barnett, M. W. & Davies, J. A. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development 128, 4329–4338 (2001).
Jain, S., Encinas, M., Johnson, E. M. Jr & Milbrandt, J. Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev. 20, 321–333 (2006).
Tang, M. J., Cai, Y., Tsai, S. J., Wang, Y. K. & Dressler, G. R. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev. Biol. 243, 128–136 (2002).
Reginensi, A. et al. SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum. Mol. Genet. 20, 1143–1153 (2011).
Lu, B. C. et al. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat. Genet. 41, 1295–1302 (2009).
Basson, M. A. et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 8, 229–239 (2005).
Michos, O. et al. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet. 6, e1000809 (2010).
Jadeja, S. et al. Identification of a new gene mutated in Fraser syndrome and mouse myelencephalic blebs. Nat. Genet. 37, 520–525 (2005).
McGregor, L. et al. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat. Genet. 34, 203–208 (2003).
Smyth, I. et al. The extracellular matrix gene Frem1 is essential for the normal adhesion of the embryonic epidermis. Proc. Natl Acad. Sci. USA 101, 13560–13565 (2004).
Takamiya, K. et al. A direct functional link between the multi-PDZ domain protein GRIP1 and the Fraser syndrome protein Fras1. Nat. Genet. 36, 172–177 (2004).
Miner, J. H. & Li, C. Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev. Biol. 217, 278–289 (2000).
Uchiyama, Y. et al. Kif26b, a kinesin family gene, regulates adhesion of the embryonic kidney mesenchyme. Proc. Natl Acad. Sci. USA 107, 9240–9245 (2010).
Linton, J. M., Martin, G. R. & Reichardt, L. F. The ECM protein nephronectin promotes kidney development via integrin α8β1-mediated stimulation of Gdnf expression. Development 134, 2501–2509 (2007).
Muller, U. et al. Integrin α8β1 is critically important for epithelial–mesenchymal interactions during kidney morphogenesis. Cell 88, 603–613 (1997).
Pitera, J. E., Scambler, P. J. & Woolf, A. S. Fras1, a basement membrane-associated protein mutated in Fraser syndrome, mediates both the initiation of the mammalian kidney and the integrity of renal glomeruli. Hum. Mol. Genet. 17, 3953–3964 (2008).
Kopan, R., Chen, S. & Little, M. Nephron progenitor cells: shifting the balance of self-renewal and differentiation. Curr. Top. Dev. Biol. 107, 293–331 (2014).
Gong, K. Q., Yallowitz, A. R., Sun, H., Dressler, G. R. & Wellik, D. M. A Hox–Eya–Pax complex regulates early kidney developmental gene expression. Mol. Cell. Biol. 27, 7661–7668 (2007).
Nie, X., Xu, J., El-Hashash, A. & Xu, P. X. Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis. Dev. Biol. 352, 141–151 (2011).
O'Brien, L. L. et al. Differential regulation of mouse and human nephron progenitors by the Six family of transcriptional regulators. Development 143, 595–608 (2016).
Boyle, S., Shioda, T., Perantoni, A. O. & de Caestecker, M. Cited1 and Cited2 are differentially expressed in the developing kidney but are not required for nephrogenesis. Dev. Dyn. 236, 2321–2330 (2007).
Hatini, V., Huh, S. O., Herzlinger, D., Soares, V. C. & Lai, E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 10, 1467–1478 (1996).
Levinson, R. S. et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132, 529–539 (2005).
Boyle, S. et al. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 313, 234–245 (2008).
Kobayashi, A. et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3, 169–181 (2008).
Kobayashi, A. et al. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Rep. 3, 650–662 (2014).
Grobstein, C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp. Cell Res. 10, 424–440 (1956).
Grobstein, C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science 118, 52–55 (1953).
Grobstein, C. Inductive interaction in the development of the mouse metanephros. J. Exp. Zool. 130, 319–339 (1955).
Rak-Raszewska, A., Hauser, P. V. & Vainio, S. Organ in vitro culture: what have we learned about early kidney development? Stem Cells Int. 2015, 959807 (2015).
Watanabe, T. & Costantini, F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev. Biol. 271, 98–108 (2004).
Sweeney, D., Lindstrom, N. & Davies, J. A. Developmental plasticity and regenerative capacity in the renal ureteric bud/collecting duct system. Development 135, 2505–2510 (2008).
Lin, Y. et al. Patterning parameters associated with the branching of the ureteric bud regulated by epithelial–mesenchymal interactions. Int. J. Dev. Biol. 47, 3–13 (2003).
Sampogna, R. V., Schneider, L. & Al-Awqati, Q. Developmental programming of branching morphogenesis in the kidney. J. Am. Soc. Nephrol. 26, 2414–2422 (2015).
Riccio, P., Cebrian, C., Zong, H., Hippenmeyer, S. & Costantini, F. Ret and Etv4 promote directed movements of progenitor cells during renal branching morphogenesis. PLoS Biol. 14, e1002382 (2016).
Packard, A. et al. Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud. Dev. Cell 27, 319–330 (2013).
Cebrian, C., Asai, N., D'Agati, V. & Costantini, F. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep. 7, 127–137 (2014).
Pepicelli, C. V., Kispert, A., Rowitch, D. H. & McMahon, A. P. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev. Biol. 192, 193–198 (1997).
Majumdar, A., Vainio, S., Kispert, A., McMahon, J. & McMahon, A. P. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–3185 (2003).
Ohuchi, H. et al. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643–649 (2000).
Varner, V. D. & Nelson, C. M. Cellular and physical mechanisms of branching morphogenesis. Development 141, 2750–2759 (2014).
Arnould, C., Lelievre-Pegorier, M., Ronco, P. & Lelongt, B. MMP9 limits apoptosis and stimulates branching morphogenesis during kidney development. J. Am. Soc. Nephrol. 20, 2171–2180 (2009).
Steer, D. L. et al. Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev. Biol. 272, 310–327 (2004).
Zent, R. et al. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev. Biol. 238, 289–302 (2001).
Zhang, X. et al. β1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136, 3357–3366 (2009).
Qiao, J., Sakurai, H. & Nigam, S. K. Branching morphogenesis independent of mesenchymal–epithelial contact in the developing kidney. Proc. Natl Acad. Sci. USA 96, 7330–7335 (1999).
Varner, V. D., Gleghorn, J. P., Miller, E., Radisky, D. C. & Nelson, C. M. Mechanically patterning the embryonic airway epithelium. Proc. Natl Acad. Sci. USA 112, 9230–9235 (2015).
Kim, H. Y. et al. Localized smooth muscle differentiation is essential for epithelial bifurcation during branching morphogenesis of the mammalian lung. Dev. Cell 34, 719–726 (2015).
Menshykau, D., Blanc, P., Unal, E., Sapin, V. & Iber, D. An interplay of geometry and signaling enables robust lung branching morphogenesis. Development 141, 4526–4536 (2014).
Cebrian, C., Borodo, K., Charles, N. & Herzlinger, D. A. Morphometric index of the developing murine kidney. Dev. Dyn. 231, 601–608 (2004).
Sims-Lucas, S. et al. Three-dimensional imaging reveals ureteric and mesenchymal defects in Fgfr2-mutant kidneys. J. Am. Soc. Nephrol. 20, 2525–2533 (2009).
Combes, A. N. et al. An integrated pipeline for the multidimensional analysis of branching morphogenesis. Nat. Protoc. 9, 2859–2879 (2014).
Short, K., Hodson, M. & Smyth, I. Spatial mapping and quantification of developmental branching morphogenesis. Development 140, 471–478 (2013).
Short, K. M., Hodson, M. J. & Smyth, I. M. Tomographic quantification of branching morphogenesis and renal development. Kidney Int. 77, 1132–1139 (2010).
Manning, G. & Krasnow, M. A. in The development of Drosophila melanogaster (eds Bate, W. & Martinez Aria, A.) 609–685 (Cold Spring Harbour Laboratory Press, 1993).
Metzger, R. J., Klein, O. D., Martin, G. R. & Krasnow, M. A. The branching programme of mouse lung development. Nature 453, 745–750 (2008).
Lamberton, T. O., Lefevre, J., Short, K. M., Smyth, I. M. & Hamilton, N. A. Comparing and distinguishing the structure of biological branching. J. Theor. Biol. 365, 226–237 (2015).
Carroll, T. J., Park, J. S., Hayashi, S., Majumdar, A. & McMahon, A. P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 (2005).
Karner, C. M. et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138, 1247–1257 (2011).
Das, A. et al. Stromal–epithelial crosstalk regulates kidney progenitor cell differentiation. Nat. Cell Biol. 15, 1035–1044 (2013).
Kispert, A., Vainio, S. & McMahon, A. P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125, 4225–4234 (1998).
Stark, K., Vainio, S., Vassileva, G. & McMahon, A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994).
Burn, S. F. et al. Calcium/NFAT signalling promotes early nephrogenesis. Dev. Biol. 352, 288–298 (2011).
Tanigawa, S. et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev. Biol. 352, 58–69 (2011).
Boyle, S. C., Kim, M., Valerius, M. T., McMahon, A. P. & Kopan, R. Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development 138, 4245–4254 (2011).
Grieshammer, U. et al. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132, 3847–3857 (2005).
Perantoni, A. O. et al. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132, 3859–3871 (2005).
Gerber, S. D., Steinberg, F., Beyeler, M., Villiger, P. M. & Trueb, B. The murine Fgfrl1 receptor is essential for the development of the metanephric kidney. Dev. Biol. 335, 106–119 (2009).
Chen, S. et al. Intrinsic age-dependent changes and cell–cell contacts regulate nephron progenitor lifespan. Dev. Cell 35, 49–62 (2015).
Barak, H. et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell 22, 1191–1207 (2012).
Rumballe, B. A. et al. Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Dev. Biol. 360, 110–122 (2011).
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1, 335–347 (1988).
Lackland, D. T., Bendall, H. E., Osmond, C., Egan, B. M. & Barker, D. J. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch. Intern. Med. 160, 1472–1476 (2000).
Hinchliffe, S. A., Sargent, P. H., Howard, C. V., Chan, Y. F. & van Velzen, D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab. Invest. 64, 777–784 (1991).
Hughson, M., Farris, A. B. III, Douglas-Denton, R., Hoy, W. E. & Bertram, J. F. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 63, 2113–2122 (2003).
Manalich, R., Reyes, L., Herrera, M., Melendi, C. & Fundora, I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 58, 770–773 (2000).
Barker, D. J. The fetal and infant origins of adult disease. BMJ 301, 1111 (1990).
Harrison, M. & Langley-Evans, S. C. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br. J. Nutr. 101, 1020–1030 (2009).
Welham, S. J., Riley, P. R., Wade, A., Hubank, M. & Woolf, A. S. Maternal diet programs embryonic kidney gene expression. Physiol. Genomics 22, 48–56 (2005).
Amri, K., Freund, N., Duong Van Huyen, J. P., Merlet-Benichou, C. & Lelievre-Pegorier, M. Altered nephrogenesis due to maternal diabetes is associated with increased expression of IGF-II/mannose-6-phosphate receptor in the fetal kidney. Diabetes 50, 1069–1075 (2001).
Lelievre-Pegorier, M. et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 54, 1455–1462 (1998).
Bauer, R. et al. Intrauterine growth restriction reduces nephron number and renal excretory function in newborn piglets. Acta Physiol. Scand. 176, 83–90 (2002).
Lisle, S. J. et al. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br. J. Nutr. 90, 33–39 (2003).
Gray, S. P., Denton, K. M., Cullen-McEwen, L., Bertram, J. F. & Moritz, K. M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 21, 1891–1902 (2010).
Kenna, K. et al. Alcohol exposure during late gestation: multiple developmental outcomes in sheep. J. Dev. Orig. Health Dis. 3, 224–236 (2012).
Wintour, E. M. et al. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J. Physiol. 549, 929–935 (2003).
Tendron, A. et al. Cyclosporin A administration during pregnancy induces a permanent nephron deficit in young rabbits. J. Am. Soc. Nephrol. 14, 3188–3196 (2003).
Komhoff, M. et al. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int. 57, 414–422 (2000).
Nathanson, S., Moreau, E., Merlet-Benichou, C. & Gilbert, T. In utero and in vitro exposure to β-lactams impair kidney development in the rat. J. Am. Soc. Nephrol. 11, 874–884 (2000).
Nicolaou, N., Renkema, K. Y., Bongers, E. M., Giles, R. H. & Knoers, N. V. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat. Rev. Nephrol. 11, 720–731 (2015).
Vivante, A. & Hildebrandt, F. Exploring the genetic basis of early-onset chronic kidney disease. Nat. Rev. Nephrol. 12, 133–146 (2016).
Skinner, M. A., Safford, S. D., Reeves, J. G., Jackson, M. E. & Freemerman, A. J. Renal aplasia in humans is associated with RET mutations. Am. J. Hum. Genet. 82, 344–351 (2008).
Weber, S. et al. SIX2 and BMP4 mutations associate with anomalous kidney development. J. Am. Soc. Nephrol. 19, 891–903 (2008).
Weber, S. et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J. Am. Soc. Nephrol. 17, 2864–2870 (2006).
Ruf, R. G. et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1–SIX1–DNA complexes. Proc. Natl Acad. Sci. USA 101, 8090–8095 (2004).
Porteous, S. et al. Primary renal hypoplasia in humans and mice with PAX2 mutations: evidence of increased apoptosis in fetal kidneys of Pax21Neu +/minus; mutant mice. Hum. Mol. Genet. 9, 1–11 (2000).
Alazami, A. M. et al. FREM1 mutations cause bifid nose, renal agenesis, and anorectal malformations syndrome. Am. J. Hum. Genet. 85, 414–418 (2009).
Vogel, M. J. et al. Mutations in GRIP1 cause Fraser syndrome. J. Med. Genet. 49, 303–306 (2012).
Humbert, C. et al. Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am. J. Hum. Genet. 94, 288–294 (2014).
Ali, A. et al. Functional characterization of GATA3 mutations causing the hypoparathyroidism-deafness-renal (HDR) dysplasia syndrome: insight into mechanisms of DNA binding by the GATA3 transcription factor. Hum. Mol. Genet. 16, 265–275 (2007).
Heidet, L. et al. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin. J. Am. Soc. Nephrol. 5, 1079–1090 (2010).
Kohlhase, J., Wischermann, A., Reichenbach, H., Froster, U. & Engel, W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat. Genet. 18, 81–83 (1998).
Sanyanusin, P. et al. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat. Genet. 9, 358–364 (1995).
Osathanondh, V. & Potter, E. L. Development of human kidney as shown by microdissection. II. Renal pelvis, calyces, and papillae. Arch. Pathol. 76, 277–289 (1963).
Peter, K. Untersuchungen uber Bau und Entwickelung der Niere (in German) (Gustav Fischer, 1909).
Neiss, W. F. Morphogenesis and histogenesis of the connecting tubule in the rat kidney. Anat. Embryol. (Berl.) 165, 81–95 (1982).
Kaissling, B. & Kriz, W. Structural analysis of the rabbit kidney. Adv. Anat. Embryol. Cell Biol. 56, 1–123 (1979).
Clissold, R. L., Hamilton, A. J., Hattersley, A. T., Ellard, S. & Bingham, C. HNF1B-associated renal and extra-renal disease — an expanding clinical spectrum. Nat. Rev. Nephrol. 11, 102–112 (2015).
Carette, C. et al. Exonic duplication of the Hepatocyte Nuclear Factor-1β gene (transcription factor 2, hepatic) as a cause of maturity onset diabetes of the young type 5. J. Clin. Endocrinol. Metab. 92, 2844–2847 (2007).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Sperber, I. Studies on the mammalian kidney. Zool. Bidrag Uppsala 22, 249–432 (1944).
Acknowledgements
We acknowledge Dr Nicholas Hamilton and Dr James Lefevre from the University of Queensland for generating Figure 4.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Morphometric analysis of branching morphogenesis during development (MP4 11012 kb)
Rights and permissions
About this article
Cite this article
Short, K., Smyth, I. The contribution of branching morphogenesis to kidney development and disease. Nat Rev Nephrol 12, 754–767 (2016). https://doi.org/10.1038/nrneph.2016.157
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.157
This article is cited by
-
Modelling Cellular Interactions and Dynamics During Kidney Morphogenesis
Bulletin of Mathematical Biology (2022)
-
Premature differentiation of nephron progenitor cell and dysregulation of gene pathways critical to kidney development in a model of preterm birth
Scientific Reports (2021)
-
Preterm birth and neonatal acute kidney injury: implications on adolescent and adult outcomes
Journal of Perinatology (2020)
-
Development of the urogenital system is regulated via the 3′UTR of GDNF
Scientific Reports (2019)