Key Points
-
The majority of the morbidity and mortality associated with diabetes mellitus is due to complications, such as diabetic kidney disease
-
A limited understanding of the underlying mechanisms responsible for the development of diabetic complications compromises efforts to develop novel strategies for treatment and prevention
-
Studies of human genetics offer powerful tools for delivering robust mechanistic insights into complex traits, such as diabetic complications, which have a substantial genetic component
-
Scientific progress to date has been limited by small sample sizes and/or phenotypic imprecision; however, several major discovery efforts are underway that have been designed to address these issues
-
The identification of genetic variants with robust effects on the risk of developing diabetic complications will accelerate efforts to develop more effective strategies for treatment and prevention
Abstract
The rising global prevalence of diabetes mellitus is accompanied by an increasing burden of morbidity and mortality that is attributable to the complications of chronic hyperglycaemia. These complications include blindness, renal failure and cardiovascular disease. Current therapeutic options for chronic hyperglycaemia reduce, but do not eradicate, the risk of these complications. Success in defining new preventative and therapeutic strategies hinges on an improved understanding of the molecular processes involved in the development of these complications. This Review explores the role of human genetics in delivering such insights, and describes progress in characterizing the sequence variants that influence individual predisposition to diabetic kidney disease, retinopathy, neuropathy and accelerated cardiovascular disease. Numerous risk variants for microvascular complications of diabetes have been reported, but very few have shown robust replication. Furthermore, only limited evidence exists of a difference in the repertoire of risk variants influencing macrovascular disease between those with and those without diabetes. Here, we outline the challenges associated with the genetic analysis of diabetic complications and highlight ongoing efforts to deliver biological insights that can drive translational benefits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alberti, K. G. & Zimmet, P. Global burden of disease—where does diabetes mellitus fit in? Nat. Rev. Endocrinol. 9, 258–260 (2013).
American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 31, 596–615 (2008).
Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (2011).
Gilg, J., Rao, A. & Fogarty, D. UK Renal Registry 16th annual report: chapter 1 UK renal replacement therapy incidence in 2012: national and centre-specific analyses. Nephron Clin. Pract. 125, 1–27 (2013).
Orchard, T. J. et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 39, 1116–1124 (1990).
Forbes, J. M. & Cooper, M. E. Mechanisms of diabetic complications. Physiol. Rev. 93, 137–188 (2013).
Fioretto, P. et al. Heterogeneous nature of microalbuminuria in NIDDM: studies of endothelial function and renal structure. Diabetologia 41, 233–236 (1998).
Pham, T. T., Sim, J. J., Kujubu, D. A., Liu, I. L. & Kumar, V. A. Prevalence of nondiabetic renal disease in diabetic patients. Am. J. Nephrol. 27, 322–328 (2007).
Wolf, G., Muller, N., Mandecka, A. & Muller, U. A. Association of diabetic retinopathy and renal function in patients with types 1 and 2 diabetes mellitus. Clin. Nephrol. 68, 81–86 (2007).
Huang, F. et al. Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: a report of 52 cases. Clin. Nephrol. 67, 293–297 (2007).
Parving, H. H. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213 (2012).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
Gilbertson, D. T. et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J. Am. Soc. Nephrol. 16, 3736–3741 (2005).
Cook, D. et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014).
Borch-Johnsen, K. et al. Is diabetic nephropathy an inherited complication? Kidney Int. 41, 719–722 (1992).
The Diabetes Control and Complications Trial Research Group. Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes 46, 1829–1839 (1997).
Quinn, M., Angelico, M. C., Warram, J. H. & Krolewski, A. S. Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 39, 940–945 (1996).
Seaquist, E. R., Goetz, F. C., Rich, S. & Barbosa, J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N. Engl. J. Med. 320, 1161–1165 (1989).
Earle, K., Walker, J., Hill, C. & Viberti, G. Familial clustering of cardiovascular disease in patients with insulin-dependent diabetes and nephropathy. N. Engl. J. Med. 326, 673–677 (1992).
Krolewski, A. S. et al. Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. N. Engl. J. Med. 318, 140–145 (1988).
Roglic, G. et al. Parental history of hypertension and parental history of diabetes and microvascular complications in insulin-dependent diabetes mellitus: the EURODIAB IDDM Complications Study. Diabet. Med. 15, 418–426 (1998).
Pettitt, D. J., Saad, M. F., Bennett, P. H., Nelson, R. G. & Knowler, W. C. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 33, 438–443 (1990).
Plenge, R. M., Scolnick, E. M. & Altshuler, D. Validating therapeutic targets through human genetics. Nat. Rev. Drug Discov. 12, 581–594 (2013).
Ritz, E., Zeng, X. X. & Rychlik, I. Clinical manifestation and natural history of diabetic nephropathy. Contrib. Nephrol. 170, 19–27 (2011).
Krolewski, A. S., Warram, J. H., Christlieb, A. R., Busick, E. J. & Kahn, C. R. The changing natural history of nephropathy in type I diabetes. Am. J. Med. 78, 785–794 (1985).
Dronavalli, S., Duka, I. & Bakris, G. L. The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 4, 444–452 (2008).
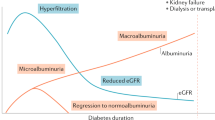
Steinke, J. M. et al. The early natural history of nephropathy in type 1 diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54, 2164–2171 (2005).
Perkins, B. A., Ficociello, L. H., Roshan, B., Warram, J. H. & Krolewski, A. S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 77, 57–64 (2010).
Nelson, R. G. et al. Incidence of end-stage renal disease in type 2 (non-insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia 31, 730–736 (1988).
Harjutsalo, V., Katoh, S., Sarti, C., Tajima, N. & Tuomilehto, J. Population-based assessment of familial clustering of diabetic nephropathy in type 1 diabetes. Diabetes 53, 2449–2454 (2004).
Langefeld, C. D. et al. Heritability of GFR and albuminuria in Caucasians with type 2 diabetes mellitus. Am. J. Kidney Dis. 43, 796–800 (2004).
Krolewski, A. S. et al. A genome-wide linkage scan for genes controlling variation in urinary albumin excretion in type II diabetes. Kidney Int. 69, 129–136 (2006).
Forsblom, C. M., Kanninen, T., Lehtovirta, M., Saloranta, C. & Groop, L. C. Heritability of albumin excretion rate in families of patients with type II diabetes. Diabetologia 42, 1359–1366 (1999).
Edwards, B. J., Haynes, C., Levenstien, M. A., Finch, S. J. & Gordon, D. Power and sample size calculations in the presence of phenotype errors for case/control genetic association studies. BMC Genet. 6, 18 (2005).
Visscher, P. M., Brown, M. A., McCarthy, M. I. & Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 90, 7–24 (2012).
Boger, C. A. & Sedor, J. R. GWAS of diabetic nephropathy: is the GENIE out of the bottle? PLoS Genet. 8, e1002989 (2012).
Ellis, J. W. et al. Validated SNPs for eGFR and their associations with albuminuria. Hum. Mol. Genet. 21, 3293–3298 (2012).
Placha, G., Canani, L. H., Warram, J. H. & Krolewski, A. S. Evidence for different susceptibility genes for proteinuria and ESRD in type 2 diabetes. Adv. Chronic Kidney Dis. 12, 155–169 (2005).
Chan, Y. et al. An excess of risk-increasing low-frequency variants can be a signal of polygenic inheritance in complex diseases. Am. J. Hum. Genet. 94, 437–452 (2014).
Gambara, V., Mecca, G., Remuzzi, G. & Bertani, T. Heterogeneous nature of renal lesions in type II diabetes. J. Am. Soc. Nephrol. 3, 1458–1466 (1993).
Ruggenenti, P. & Remuzzi, G. Nephropathy of type 1 and type 2 diabetes: diverse pathophysiology, same treatment? Nephrol. Dial. Transplant. 15, 1900–1902 (2000).
Kottgen, A. et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 41, 712–717 (2009).
Barreiro, L. B., Laval, G., Quach, H., Patin, E. & Quintana-Murci, L. Natural selection has driven population differentiation in modern humans. Nat. Genet. 40, 340–345 (2008).
Genovese, G. et al. Association of trypanolytic APOL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Palmer, N. D. et al. Evaluation of candidate nephropathy susceptibility genes in a genome-wide association study of African American diabetic kidney disease. PLoS ONE 9, e88273 (2014).
Parsa, A. et al. APOL1 risk variants, race, and progression of chronic kidney disease. N. Engl. J. Med. 369, 2183–2196 (2013).
Mooyaart, A. L. et al. Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia 54, 544–553 (2011).
Nikzamir, A., Nakhjavani, M., Esteghamati, A. & Rashidi, A. Correlates of ACE activity in macroalbuminuric type 2 diabetic patients treated with chronic ACE inhibition. Nephrol. Dial. Transplant. 23, 1274–1277 (2008).
Rigat, B. et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 86, 1343–1346 (1990).
Wang, F. et al. Association between genetic polymorphism of the angiotensin-converting enzyme and diabetic nephropathy: a meta-analysis comprising 26,580 subjects. J. Renin Angiotensin Aldosterone Syst. 13, 161–174 (2012).
Germain, M. et al. SORBS1 gene, a new candidate for diabetic nephropathy: results from a multi-stage genome-wide association study in patients with type 1 diabetes. Diabetologia 58, 543–548 (2014).
Hoggart, C. J., Clark, T. G., De Iorio, M., Whittaker, J. C. & Balding, D. J. Genome-wide significance for dense SNP and resequencing data. Genet. Epidemiol. 32, 179–185 (2008).
Thompson, J. R., Attia, J. & Minelli, C. The meta-analysis of genome-wide association studies. Brief Bioinform. 12, 259–269 (2011).
Mahajan, A. et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 46, 234–244 (2014).
Deloukas, P. et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45, 25–33 (2013).
Craig, D. W., Millis, M. P. & DiStefano, J. K. Genome-wide SNP genotyping study using pooled DNA to identify candidate markers mediating susceptibility to end-stage renal disease attributed to type 1 diabetes. Diabet. Med. 26, 1090–1098 (2009).
Pezzolesi, M. G. et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58, 1403–1410 (2009).
Williams, W. W. et al. Association testing of previously reported variants in a large case-control meta-analysis of diabetic nephropathy. Diabetes 61, 2187–2194 (2012).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Sandholm, N. et al. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 8, e1002921 (2012).
Schelling, J. R. et al. Genome-wide scan for estimated glomerular filtration rate in multi-ethnic diabetic populations: the Family Investigation of Nephropathy and Diabetes (FIND). Diabetes 57, 235–243 (2008).
Igo, R. P. Jr et al. Genomewide linkage scan for diabetic renal failure and albuminuria: the FIND study. Am. J. Nephrol. 33, 381–389 (2011).
Thameem, F. et al. A genome-wide search for linkage of estimated glomerular filtration rate (eGFR) in the Family Investigation of Nephropathy and Diabetes (FIND). PLoS ONE 8, e81888 (2013).
McDonough, C. W. et al. A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Int. 79, 563–572 (2011).
Rayner, N. W. et al. Abstracts of the 74th Scientific Sessions of the American Diabetes Association. Whole-exome sequencing in type 1 diabetic nephropathy. Diabetes 63 (Suppl. 1), A37 (2014).
Bonomo, J. A. et al. Coding variants in nephrin (NPHS1) and susceptibility to nephropathy in African Americans. Clin. J. Am. Soc. Nephrol. 9, 1434–1440 (2014).
Majithia, A. R. et al. Rare variants in PPARG with decreased activity in adipocyte differentiation are associated with increased risk of type 2 diabetes. Proc. Natl Acad. Sci. USA 111, 13127–13132 (2014).
Rani, P. K. et al. Albuminuria and diabetic retinopathy in type 2 diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology And Molecular Genetic Study (SN-DREAMS, report 12). Diabetol. Metab. Syndr. 3, 9 (2011).
Drury, P. L. et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 54, 32–43 (2011).
Groop, P. H. et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58, 1651–1658 (2009).
Viswanath, K. & McGavin, D. D. Diabetic retinopathy: clinical findings and management. Community Eye Health 16, 21–24 (2003).
Yau, J. W. et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564 (2012).
Arar, N. H. et al. Heritability of the severity of diabetic retinopathy: the FIND-Eye study. Invest. Ophthalmol. Vis. Sci. 49, 3839–3845 (2008).
Hietala, K., Forsblom, C., Summanen, P., Groop, P. H. & FinnDiane Study Group. Heritability of proliferative diabetic retinopathy. Diabetes 57, 2176–2180 (2008).
Looker, H. C. et al. Genome-wide linkage analyses to identify loci for diabetic retinopathy. Diabetes 56, 1160–1166 (2007).
Cho, H. & Sobrin, L. Genetics of diabetic retinopathy. Curr. Diab. Rep. 14, 515 (2014).
Sobrin, L. et al. Candidate gene association study for diabetic retinopathy in persons with type 2 diabetes: the Candidate Gene Association Resource (CARe). Invest. Ophthalmol. Vis. Sci. 52, 7593–7602 (2011).
Awata, T. et al. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes 51, 1635–1639 (2002).
Stevens, A., Soden, J., Brenchley, P. E., Ralph, S. & Ray, D. W. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res. 63, 812–816 (2003).
Qiu, M., Xiong, W., Liao, H. & Li, F. VEGF −634G>C polymorphism and diabetic retinopathy risk: a meta-analysis. Gene 518, 310–315 (2013).
Zhao, T. & Zhao, J. Association between the −634C/G polymorphisms of the vascular endothelial growth factor and retinopathy in type 2 diabetes: a meta-analysis. Diabetes Res. Clin. Pract. 90, 45–53 (2010).
Abhary, S., Hewitt, A. W., Burdon, K. P. & Craig, J. E. A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes 58, 2137–2147 (2009).
Abhary, S. et al. Aldose reductase gene polymorphisms and diabetic retinopathy susceptibility. Diabetes Care 33, 1834–1836 (2010).
Tong, Z. et al. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc. Natl Acad. Sci. USA 105, 6998–7003 (2008).
Sheu, W. H. et al. Genome-wide association study in a Chinese population with diabetic retinopathy. Hum. Mol. Genet. 22, 3165–3173 (2013).
Huang, Y. C. et al. Genome-wide association study of diabetic retinopathy in a Taiwanese population. Ophthalmology 118, 642–648 (2011).
Grassi, M. A. et al. Genome-wide meta-analysis for severe diabetic retinopathy. Hum. Mol. Genet. 20, 2472–2481 (2011).
Grassi, M. A. et al. Replication analysis for severe diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 53, 2377–2381 (2012).
Said, G. Diabetic neuropathy—a review. Nat. Clin. Pract. Neurol. 3, 331–340 (2007).
Bennett, D. L. H. & Woods, C. G. Painful and painless channelopathies. Lancet Neurol. 13, 587–599 (2014).
Ciccacci, C. et al. Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol. 51, 663–671 (2014).
Barakat, K. & Hitman, G. A. Genetic susceptibility to macrovascular complications of type 2 diabetes mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 15, 359–370 (2001).
Beckman, J. A., Creager, M. A. & Libby, P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287, 2570–2581 (2002).
Waller, B. F., Palumbo, P. J., Lie, J. T. & Roberts, W. C. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am. J. Med. 69, 498–506 (1980).
Martini, S. R. & Kent, T. A. Hyperglycaemia in acute ischemic stroke: a vascular perspective. J. Cereb. Blood Flow Metab. 27, 435–451 (2007).
Prakash, R. et al. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke 44, 2875–2882 (2013).
Chen, Q., Smith, C. Y., Bailey, K. R., Wennberg, P. W. & Kullo, I. J. Disease location is associated with survival in patients with peripheral arterial disease. J. Am. Heart Assoc. 2, e000304 (2013).
Gschwendtner, A. et al. Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann. Neurol. 65, 531–539 (2009).
International Stroke Genetics Consortium et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat. Genet. 44, 328–333 (2012).
Abboud, S. et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) gene is a risk factor of large-vessel atherosclerosis stroke. PLoS ONE 2, e1043 (2007).
Samani, N. J. et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 357, 443–453 (2007).
Thorgeirsson, T. E. et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452, 638–642 (2008).
Murabito, J. M. et al. Association between chromosome 9p21 variants and the ankle-brachial index identified by a meta-analysis of 21 genome-wide association studies. Circ. Cardiovasc. Genet. 5, 100–112 (2012).
Hopewell, J. C. et al. Lipoprotein(a) genetic variants associated with coronary and peripheral vascular disease but not with stroke risk in the Heart Protection Study. Circ. Cardiovasc. Genet. 4, 68–73 (2011).
Van Zuydam, N. R. Abstracts of the 49th Annual Meeting of the EASD: known SNPs in ADAMTS7, the 9p21 region and UBE2E interact with type 2 diabetes status to modify the risk of coronary artery disease in large populations. Diabetologia 56 (Suppl. 1), S76–S77 (2013).
Doria, A. et al. Interaction between poor glycaemic control and 9p21 locus on risk of coronary artery disease in type 2 diabetes. JAMA 300, 2389–2397 (2008).
Qi, L. et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA 310, 821–828 (2013).
Zeggini, E. et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316, 1336–1341 (2007).
Morris, A. et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44, 981–990 (2012).
Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316, 1331–1336 (2007).
Scott, L. J. et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316, 1341–1345 (2007).
Shea, J. et al. Comparing strategies to fine-map the association of common SNPs at chromosome 9p21 with type 2 diabetes and myocardial infarction. Nat. Genet. 43, 801–805 (2011).
Rivera, N. V. et al. Assessment of the 9p21.3 locus in severity of coronary artery disease in the presence and absence of type 2 diabetes. BMC Med. Genet. 14, 11 (2013).
Writing Team for the Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 287, 2563–2569 (2002).
Writing Team for the Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290, 2159–2167 (2003).
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
Kato, M. & Natarajan, R. Diabetic nephropathy—emerging epigenetic mechanisms. Nat. Rev. Nephrol. 10, 517–530 (2014).
Reddy, M. A. & Natarajan, R. Epigenetics in diabetic kidney disease. J. Am. Soc. Nephrol. 22, 2182–2185 (2011).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
Zhang, Q. et al. Gene expression profiling in glomeruli of diabetic nephropathy rat. Exp. Biol. Med. (Maywood) 237, 903–911 (2012).
Lee, Y. J. et al. E3 ubiquitin-protein ligases in rat kidney collecting duct: response to vasopressin stimulation and withdrawal. Am. J. Physiol. Renal Physiol. 301, F883–F896 (2011).
Song, Y., Ailenberg, M. & Silverman, M. Human munc13 is a diacylglycerol receptor that induces apoptosis and may contribute to renal cell injury in hyperglycaemia. Mol. Biol. Cell 10, 1609–1619 (1999).
Tregouet, D. A. et al. G/T substitution in intron 1 of the UNC13B gene is associated with increased risk of nephropathy in patients with type 1 diabetes. Diabetes 57, 2843–2850 (2008).
Bell, C. G. et al. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med. Genomics 3, 33 (2010).
International Consortium for Blood Pressure Genome-Wide Association Studies et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011).
Franceschini, N. et al. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am. J. Hum. Genet. 93, 545–554 (2013).
Tomino, Y., Cooper, M. E., Kurtz, T. W. & Shimizu, Y. Experimental models of type-2 diabetic nephropathy. Exp. Diabetes Res. 2012, 218917 (2012).
Engelbertsen, D. et al. Increased inflammation in atherosclerotic lesions of diabetic Akita-LDLr−/− mice compared to nondiabetic LDLr−/− mice. Exp. Diabetes Res. 2012, 176162 (2012).
Brosius, F. C. 3rd et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 20, 2503–2512 (2009).
Hsueh, W. et al. Recipes for creating animal models of diabetic cardiovascular disease. Circ. Res. 100, 1415–1427 (2007).
Woroniecka, K. I. et al. Transcriptome analysis of human diabetic kidney disease. Diabetes 60, 2354–2369 (2011).
Chen, H. H., Almontashiri, N. A., Antoine, D. & Stewart, A. F. Functional genomics of the 9p21.3 locus for atherosclerosis: clarity or confusion? Curr. Cardiol. Rep. 16, 502 (2014).
CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45, 25–33 (2013).
Nathan, D. M. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353, 2643–2653 (2005).
Berndt, S. I. et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 45, 501–512 (2013).
Mueller, P. W. et al. Genetics of Kidneys in Diabetes (GoKinD) study: a genetics collection available for identifying genetic susceptibility factors for diabetic nephropathy in type 1 diabetes. J. Am. Soc. Nephrol. 17, 1782–1790 (2006).
Scott, R. A. et al. Large-scale association analyses identify new loci influencing glycaemic traits and provide insight into the underlying biological pathways. Nat. Genet. 44, 991–1005 (2012).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article, and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
E.A. and N.R.V.Z. have received salary support, and L.C.G. and M.I.M, have received research support, from the Innovative Medicines Initiative and the Juvenile Diabetes Research Foundation for research on the genetic basis of diabetic complications.
Rights and permissions
About this article
Cite this article
Ahlqvist, E., van Zuydam, N., Groop, L. et al. The genetics of diabetic complications. Nat Rev Nephrol 11, 277–287 (2015). https://doi.org/10.1038/nrneph.2015.37
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.37
This article is cited by
-
Nidogen-1 could play a role in diabetic kidney disease development in type 2 diabetes: a genome-wide association meta-analysis
Human Genomics (2022)
-
Elevated testicular apoptosis is associated with elevated sphingosine driven by gut microbiota in prediabetic sheep
BMC Biology (2022)
-
Genome-wide association studies: assessing trait characteristics in model and crop plants
Cellular and Molecular Life Sciences (2021)
-
Serum lncRNA HAND2-AS1 is downregulated in diabetic patients with chronic renal failure and ameliorates cell apoptosis
Diabetology & Metabolic Syndrome (2020)
-
Genetics of diabetes mellitus and diabetes complications
Nature Reviews Nephrology (2020)