Key Points
-
The innate immune system consists of several classes of pattern recognition receptors, including Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), which detect pathogen-associated and danger-associated molecular patterns and initiate an inflammatory response
-
TLR2 and TLR4 are the predominant TLRs expressed on pancreatic β cells which trigger an inflammatory response that results in insulitis during type 1 diabetes mellitus
-
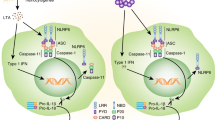
TLR2 and TLR4 signalling, and activation of NLRP3 inflammasomes result in the production of various proinflammatory cytokines that can induce insulin resistance in type 2 diabetes mellitus (T2DM) and obesity
-
Innate immune responses in T2DM and obesity are modulated by the status of the gut microbiota, autophagy, and adipokines
-
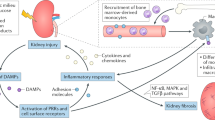
TLR2, TLR4, NOD2, and NLRP3 inflammasome-mediated inflammation are involved in the perpetuation of inflammation in diabetic nephropathy
-
The activation of TLRs and NLRs stimulates the expression of MCP-1, which is associated with the progression of diabetic nephropathy
Abstract
The innate immune system includes several classes of pattern recognition receptors (PRRs), including membrane-bound Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). These receptors detect pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) in the extracellular and intracellular space. Intracellular NLRs constitute inflammasomes, which activate and release caspase-1, IL-1β, and IL-18 thereby initiating an inflammatory response. Systemic and local low-grade inflammation and release of proinflammatory cytokines are implicated in the development and progression of diabetes mellitus and diabetic nephropathy. TLR2, TLR4, and the NLRP3 inflammasome can induce the production of various proinflammatory cytokines and are critically involved in inflammatory responses in pancreatic islets, and in adipose, liver and kidney tissues. This Review describes how innate immune system-driven inflammatory processes can lead to apoptosis, tissue fibrosis, and organ dysfunction resulting in insulin resistance, impaired insulin secretion, and renal failure. We propose that careful targeting of TLR2, TLR4, and NLRP3 signalling pathways could be beneficial for the treatment of diabetes mellitus and diabetic nephropathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas, 6th edn. Brussels, Belgium: International Diabetes Federation [online], (2013).
Saran, R. et al. US renal data system 2014 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 66, S1–S306 (2015).
Gaede, P., Lund-Andersen, H., Parving, H. H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591 (2008).
Wada, J. & Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 124, 139–152 (2013).
Murphy, K., Travers, P., Walport, M. & Janeway, C. Janeway's Immunobiology 8th edn (Garland Science, 2012).
Mogensen, T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273 (2009).
Dowling, J. K. & O'Neill, L. A. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol. 47, 424–443 (2012).
Leemans, J. C., Kors, L., Anders, H. J. & Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 10, 398–414 (2014).
Anders, H. J. & Muruve, D. A. The inflammasomes in kidney disease. J. Am. Soc. Nephrol. 22, 1007–1018 (2011).
Prajapati, B., Jena, P. K., Rajput, P., Purandhar, K. & Seshadri, S. Understanding and modulating the Toll like receptors (TLRs) and NOD like receptors (NLRs) cross talk in type 2 diabetes. Curr. Diabetes Rev. 10, 190–200 (2014).
Takeda, K. & Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 (2005).
Godfroy, J. I. 3rd, Roostan, M., Moroz, Y. S., Korendovych, I. V. & Yin, H. Isolated Toll-like receptor transmembrane domains are capable of oligomerization. PLoS ONE 7, e48875 (2012).
Janssens, S. & Beyaert, R. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16, 637–646 (2003).
Xu, Y. et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408, 111–115 (2000).
Song, D. H. & Lee, J. O. Sensing of microbial molecular patterns by Toll-like receptors. Immunol. Rev. 250, 216–229 (2012).
Liu, Y., Yin, H., Zhao, M. & Lu, Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin. Rev. Allergy Immunol. 47, 136–147 (2014).
De Nardo, D. Toll-like receptors: activation, signalling and transcriptional modulation. Cytokine 74, 181–189 (2015).
Frazão, J. B., Errante, P. R. & Condino-Neto, A. Toll-like receptors' pathway disturbances are associated with increased susceptibility to infections in humans. Arch. Immunol. Ther. Exp. (Warsz.) 61, 427–443 (2013).
Kawasaki, T. & Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 5, 461 (2014).
Nakamura, N. et al. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 509, 240–244 (2014).
Bertrand, M. J. et al. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30, 789–801 (2009).
Lamkanfi, M. & Dixit, V. M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 227, 95–105 (2009).
Caruso, R., Warner, N., Inohara, N. & Nunez, G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41, 898–908 (2014).
Arend, W. P., Palmer, G. & Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 (2008).
Zhong, Y., Kinio, A. & Saleh, M. Functions of NOD-like receptors in human diseases. Front. Immunol. 4, 333 (2013).
Bauernfeind, F. & Hornung, V. Of inflammasomes and pathogens—sensing of microbes by the inflammasome. EMBO Mol. Med. 5, 814–826 (2013).
Vanaja, S. K., Rathinam, V. A. & Fitzgerald, K. A. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 25, 308–315 (2015).
Guo, H., Callaway, J. B. & Ting, J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 21, 677–687 (2015).
Walker, L. S. & von Herrath, M. CD4 T cell differentiation in type 1 diabetes. Clin. Exp. Immunol. http://dx.doi.org/10.1111/cei.12672.
Zipris, D. Innate immunity and its role in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 15, 326–331 (2008).
Park, Y., Park, S., Yoo, E., Kim, D. & Shin, H. Association of the polymorphism for Toll-like receptor 2 with type 1 diabetes susceptibility. Ann. NY Acad. Sci. 1037, 170–174 (2004).
Nussbaum, G., Zanin-Zhorov, A., Quintana, F., Lider, O. & Cohen, I. R. Peptide p277 of HSP60 signals T cells: inhibition of inflammatory chemotaxis. Int. Immunol. 18, 1413–1419 (2006).
Karumuthil-Melethil, S. et al. TLR2- and Dectin 1-associated innate immune response modulates T-cell response to pancreatic β-cell antigen and prevents type 1 diabetes. Diabetes 64, 1341–1357 (2015).
Karumuthil-Melethil, S., Perez, N., Li, R. & Vasu, C. Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J. Immunol. 181, 8323–8334 (2008).
Filippi, C. M. et al. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur. J. Immunol. 41, 1399–1409 (2011).
Lee, M. S. Treatment of autoimmune diabetes by inhibiting the initial event. Immune Netw. 13, 194–198 (2013).
Li, M., Song, L., Gao, X., Chang, W. & Qin, X. Toll-like receptor 4 on islet β cells senses expression changes in high-mobility group BOX 1 and contributes to the initiation of type 1 diabetes. Exp. Mol. Med. 44, 260–267 (2012).
Kruger, B. et al. Islet-expressed TLR2 and TLR4 sense injury and mediate early graft failure after transplantation. Eur. J. Immunol. 40, 2914–2924 (2010).
Xiao, L., Liu, Y. & Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 306, H317–H325 (2014).
Li, J. et al. Enhanced inflammatory responses to toll-like receptor 2/4 stimulation in type 1 diabetic coronary artery endothelial cells: the effect of insulin. Cardiovasc. Diabetol. 9, 90 (2010).
Schmidt, L. & Carrillo-Sepulveda, M. A. Toll-like receptor 2 mediates vascular contraction and activates RhoA signaling in vascular smooth muscle cells from STZ-induced type 1 diabetic rats. Pflugers Archiv. http://dx.doi.org/10.1007/s00424-015-1688–2.
Labrum, R., Bevan, S., Sitzer, M., Lorenz, M. & Markus, H. S. Toll receptor polymorphisms and carotid artery intima-media thickness. Stroke 38, 1179–1184 (2007).
Liu, F. et al. Frequency of TLR 2, 4, and 9 gene polymorphisms in Chinese population and their susceptibility to type 2 diabetes and coronary artery disease. J. Biomed. Biotechnol. 2012, 373945 (2012).
Martinon, F., Gaide, O., Petrilli, V., Mayor, A. & Tschopp, J. NALP inflammasomes: a central role in innate immunity. Semin. Immunopathol. 29, 213–229 (2007).
Sarkar, S. A. et al. Cytokine-mediated induction of anti-apoptotic genes that are linked to nuclear factor κ-B (NF-κB) signalling in human islets and in a mouse β cell line. Diabetologia 52, 1092–1101 (2009).
Moran, A. et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 381, 1905–1915 (2013).
Pontillo, A. et al. Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity 43, 583–589 (2010).
Motta, V. N. et al. Identification of the inflammasome Nlrp1b as the candidate gene conferring diabetes risk at the Idd4.1 locus in the nonobese diabetic mouse. J. Immunol. 194, 5663–5673 (2015).
Fresno, M., Alvarez, R. & Cuesta, N. Toll-like receptors, inflammation, metabolism and obesity. Arch. Pysiol. Biochem. 117, 151–164 (2011).
Tanti, J. F., Ceppo, F., Jager, J. & Berthou, F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 3, 181 (2012).
Apostolopoulos, V. et al. The complex immunological and inflammatory network of adipose tissue in obesity. Mol. Nutr. Food Res. http://dx.doi.org/10.1002/mnfr.201500272.
Himes, R. W. & Smith, C. W. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 24, 731–739 (2010).
Davis, J. E., Braucher, D. R., Walker-Daniels, J. & Spurlock, M. E. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J. Nutr. Biochem. 22, 136–141 (2011).
Kuo, L. H. et al. Toll-like receptor 2 deficiency improves insulin sensitivity and hepatic insulin signalling in the mouse. Diabetologia 54, 168–179 (2011).
Vitseva, O. I. et al. Inducible Toll-like receptor and NF-κB regulatory pathway expression in human adipose tissue. Obesity 16, 932–937 (2008).
Murumalla, R. K. et al. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 11, 175 (2012).
Wan, Z., Durrer, C., Mah, D., Simtchouk, S. & Little, J. P. One-week high-fat diet leads to reduced Toll-like receptor 2 expression and function in young healthy men. Nutrition Res. 34, 1045–1051 (2014).
Hosoi, T., Yokoyama, S., Matsuo, S., Akira, S. & Ozawa, K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS ONE 5, e12537 (2010).
Bollyky, P. L. et al. The Toll-like receptor signaling molecule Myd88 contributes to pancreatic β-cell homeostasis in response to injury. PLoS ONE 4, e5063 (2009).
Caesar, R., Tremaroli, V., Kovatcheva-Datchary, P., Cani, P. D. & Backhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 22, 1–11 (2015).
Kleinridders, A. et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259 (2009).
Alkanani, A. K. et al. Induction of diabetes in the RIP-B7.1 mouse model is critically dependent on TLR3 and MyD88 pathways and is associated with alterations in the intestinal microbiome. Diabetes 63, 619–631 (2014).
Everard, A. et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat. Comm. 5, 5648 (2014).
Balmer, M. L. et al. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J. Immunol. 193, 5273–5283 (2014).
Schenten, D. et al. Signaling through the adaptor molecule MyD88 in CD4+ T cells is required to overcome suppression by regulatory T cells. Immunity 40, 78–90 (2014).
Velloso, L. A., Folli, F. & Saad, M. J. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocrine Rev. 36, 245–271 (2015).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Tsukumo, D. M. et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56, 1986–1998 (2007).
Suganami, T. et al. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem. Biophys. Res. Comm. 354, 45–49 (2007).
Poggi, M. et al. C3H/HeJ mice carrying a Toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50, 1267–1276 (2007).
Frisard, M. I. et al. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am. J. Physiol. Endocrinol. Metab. 298, E988–E998 (2010).
Kim, F. et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 100, 1589–1596 (2007).
Saberi, M. et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Jia, L. et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Comm. 5, 3878 (2014).
Kiely, A., Robinson, A., McClenaghan, N. H., Flatt, P. R. & Newsholme, P. Toll-like receptor agonist induced changes in clonal rat BRIN-BD11 β-cell insulin secretion and signal transduction. J. Endocrinol. 202, 365–373 (2009).
Hardy, O. T., Kim, A., Ciccarelli, C., Hayman, L. L. & Wiecha, J. Increased Toll-like receptor (TLR) mRNA expression in monocytes is a feature of metabolic syndrome in adolescents. Pediatr. Obes. 8, e19–e23 (2013).
Samuvel, D. J. et al. TLR4 activation and IL-6-mediated cross talk between adipocytes and mononuclear cells synergistically stimulate MMP-1 expression. Endocrinology 152, 4662–4671 (2011).
Nativel, B. et al. Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: contribution to chronic inflammation in fat tissue. PLoS ONE 8, e76039 (2013).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Nagareddy, P. R. et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 19, 821–835 (2014).
Hussey, S. E. et al. TAK-242, a small-molecule inhibitor of Toll-like receptor 4 signalling, unveils similarities and differences in lipopolysaccharide- and lipid-induced inflammation and insulin resistance in muscle cells. Biosci. Rep. 33, 37–47 (2013).
Caricilli, A. M. et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 9, e1001212 (2011).
Kellermayer, R. et al. Colonic mucosal DNA methylation, immune response, and microbiome patterns in Toll-like receptor 2-knockout mice. FASEB J. 25, 1449–1460 (2011).
Kim, K. A., Gu, W., Lee, I. A., Joh, E. H. & Kim, D. H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 7, e47713 (2012).
Ji, Y. et al. Diet-induced alterations in gut microflora contribute to lethal pulmonary damage in TLR2/TLR4-deficient mice. Cell Rep. 8, 137–149 (2014).
Vijay-Kumar, M. et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2010).
Pekkala, S. et al. Toll-like receptor 5 in obesity: the role of gut microbiota and adipose tissue inflammation. Obesity 23, 581–590 (2015).
Leichtle, A. et al. TLR4-mediated induction of TLR2 signaling is critical in the pathogenesis and resolution of otitis media. Innate Immun. 15, 205–215 (2009).
Coope, A. et al. Chaperone insufficiency links TLR4 protein signaling to endoplasmic reticulum stress. J. Biol. Chem. 287, 15580–15589 (2012).
Okla, M. et al. Activation of Toll-like receptor (TLR) 4 attenuates adaptive thermogenesis via endoplasmic reticulum stress. J. Biol. Chem. http://dx.doi.org/10.1074/jbc.M115.677724.
Oslowski, C. M. et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 16, 265–273 (2012).
Lerner, A. G. et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264 (2012).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Kim, S. et al. Endoplasmic reticulum stress is sufficient for the induction of IL-1β production via activation of the NF-κB and inflammasome pathways. Innate Immun. 20, 799–815 (2014).
Kim, M. S., Choi, M. S. & Han, S. N. High fat diet-induced obesity leads to proinflammatory response associated with higher expression of NOD2 protein. Nutr. Res. Pract. 5, 219–223 (2011).
Denou, E. et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol. Med. 7, 259–274 (2015).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Youm, Y. H. et al. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology 152, 4039–4045 (2011).
Westwell-Roper, C., Nackiewicz, D., Dan, M. & Ehses, J. A. Toll-like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunol. Cell Biol. 92, 314–323 (2014).
Jourdan, T. et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates β cell loss in type 2 diabetes. Nat. Med. 19, 1132–1140 (2013).
Zambetti, L. P. & Mortellaro, A. NLRPs, microbiota, and gut homeostasis: unravelling the connection. J. Pathol. 233, 321–330 (2014).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
O'Connor, J. C. et al. IL-1β-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J. Immunol. 174, 4991–4997 (2005).
Mitroulis, I., Skendros, P. & Ritis, K. Targeting IL-1β in disease; the expanding role of NLRP3 inflammasome. Eur. J. Int. Med. 21, 157–163 (2010).
McGettrick, A. F. & O'Neill, L. A. NLRP3 and IL-1β in macrophages as critical regulators of metabolic diseases. Diabetes Obesity Metab. 15, S19–S25 (2013).
Freigang, S. et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 14, 1045–1053 (2013).
Hara, N., Alkanani, A. K., Dinarello, C. A. & Zipris, D. Modulation of virus-induced innate immunity and type 1 diabetes by IL-1 blockade. Innate Immun. 20, 574–584 (2013).
Finucane, O. M. et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 64, 2116–2128 (2015).
Chen, K. et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int. J. Biochem. Cell Biol. 45, 932–943 (2013).
Nackiewicz, D. et al. TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair β cell insulin gene expression via IL-1 and IL-6. Diabetologia 57, 1645–1654 (2014).
Dalmas, E. et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes 63, 1966–1977 (2014).
Gottlieb, P. A. et al. α1-antitrypsin therapy downregulates toll-like receptor-induced IL-1β responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 99, E1418–E1426 (2014).
Wang, F. et al. Losartan inhibits LPS + ATP-induced IL-1β secretion from mouse primary macrophages by suppressing NALP3 inflammasome. Pharmazie 69, 680–684 (2014).
Martinez-Micaelo, N., González-Abuín, N., Pinent, M., Ardévol, A. & Blay, M. Procyanidin B2 inhibits inflammasome-mediated IL-1β production in lipopolysaccharide-stimulated macrophages. Mol. Nutr. Food Res. 59, 262–269 (2015).
Miao, H. et al. Macrophage CGI-58 deficiency promotes IL-1β transcription by activating the SOCS3–FOXO1 pathway. Clin. Sci. 128, 493–506 (2015).
Salminen, A., Kaarniranta, K. & Kauppinen, A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging 4, 166–175 (2012).
Lim, Y. M. et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat. Comm. 5, 4934 (2014).
Liu, K. et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 11, 271–284 (2015).
Fasshauer, M. & Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 36, 461–470 (2015).
Crujeiras, A. B. et al. Leptin resistance in obesity: an epigenetic landscape. Life Sci. http://dx.doi.org/10.1016/j.lfs.2015.05.003.
Jitprasertwong, P., Jaedicke, K. M., Nile, C. J., Preshaw, P. M. & Taylor, J. J. Leptin enhances the secretion of interleukin (IL)-18, but not IL-1β, from human monocytes via activation of caspase-1. Cytokine 65, 222–230 (2014).
Owen, B. M., Mangelsdorf, D. J. & Kliewer, S. A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 26, 22–29 (2015).
Asrih, M., Altirriba, J., Rohner-Jeanrenaud, F. & Jornayvaz, F. R. Ketogenic diet impairs FGF21 signaling and promotes differential inflammatory responses in the liver and white adipose tissue. PLoS ONE 10, e0126364 (2015).
Liu, M. H. FGF-21 alleviates diabetes-associated vascular complications: inhibiting NF-κB/NLRP3 inflammasome-mediated inflammation? Int. J. Cardiol. 185, 320–321 (2015).
Roberts, R. L. et al. Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis 218, 123–126 (2011).
Li, Y., Xu, S., Jiang, B., Cohen, R. A. & Zang, M. Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS ONE 8, e67532 (2013).
Zheng, F., Xing, S., Gong, Z. & Xing, Q. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ. 22, 746–750 (2013).
Anders, H. J. & Lech, M. NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int. 84, 225–228 (2013).
Mudaliar, H., Pollock, C. & Panchapakesan, U. Role of Toll-like receptors in diabetic nephropathy. Clin. Sci. 126, 685–694 (2014).
Pillon, N. J. et al. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promotes monocyte adhesion and impairs insulin transcytosis. Am. J. Physiol. Endocrinol. Metab. 309, E35–E44 (2015).
Rajamani, U. & Jialal, I. Hyperglycemia induces Toll-like receptor-2 and -4 expression and activity in human microvascular retinal endothelial cells: implications for diabetic retinopathy. J. Diabetes Res. 2014, 790902 (2014).
Tang, S. C., Leung, J. C. & Lai, K. N. Diabetic tubulopathy: an emerging entity. Contrib. Nephrol. 170, 124–134 (2011).
Mudaliar, H. et al. The role of TLR2 and 4-mediated inflammatory pathways in endothelial cells exposed to high glucose. PLoS ONE 9, e108844 (2014).
Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 57, 1428–1434 (2001).
Salmela, K. et al. A randomized multicenter trial of the anti-ICAM-1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti-ICAM-1 Renal Transplant Study Group. Transplantation 67, 729–736 (1999).
Musi, N. Phase 2 study of the role of pharmacologic inhibition of TLR4 with E5564 on glucose metabolism in insulin resistant subjects. ClinicalTrials.gov [online].
Devaraj, S. et al. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler. Thromb. Vasc. Biol. 31, 1796–1804 (2011).
Sawa, Y., Takata, S., Hatakeyama, Y., Ishikawa, H. & Tsuruga, E. Expression of toll-like receptor 2 in glomerular endothelial cells and promotion of diabetic nephropathy by Porphyromonas gingivalis lipopolysaccharide. PLoS ONE 9, e97165 (2014).
Mudaliar, H. et al. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am. J. Physiol. Renal Physiol. 305, F143–F154 (2013).
Kaur, H., Chien, A. & Jialal, I. Hyperglycemia induces Toll like receptor 4 expression and activity in mouse mesangial cells: relevance to diabetic nephropathy. Am. J. Physiol. Renal Physiol. 303, F1145–F1150 (2012).
Yang, M. et al. Proinflammatory CD14+CD16+ monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation 35, 388–396 (2012).
Cha, J. J. et al. Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology 154, 2144–2155 (2013).
Lin, M. et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 83, 887–900 (2013).
Jialal, I., Major, A. M. & Devaraj, S. Global Toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. J. Diabetes Complications 28, 755–761 (2014).
Saurus, P. et al. Podocyte apoptosis is prevented by blocking the Toll-like receptor pathway. Cell Death Dis. 6, e1752 (2015).
Ma, J. et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS ONE 9, e97985 (2014).
Verzola, D. et al. Enhanced glomerular Toll-like receptor 4 expression and signaling in patients with type 2 diabetic nephropathy and microalbuminuria. Kidney Int. 86, 1229–1243 (2014).
Oh, D. J., Kim, H. R., Lee, M. K. & Woo, Y. S. Profile of human β-defensins 1,2 and proinflammatory cytokines (TNF-α, IL-6) in patients with chronic kidney disease. Kidney Blood Press. Res. 37, 602–610 (2013).
Navarro-González, J. F., Jarque, A., Muros, M., Mora, C. & García, J. Tumor necrosis factor-α as a therapeutic target for diabetic nephropathy. Cytokine Growth Factor Rev. 20, 165–173 (2009).
Navarro, J. F. & Mora-Fernández, C. The role of TNF-α in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 17, 441–450 (2006).
Du, P. et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int. 84, 265–276 (2013).
Shang, J. et al. Identification of NOD2 as a novel target of RNA-binding protein HuR: evidence from NADPH oxidase-mediated HuR signaling in diabetic nephropathy. Free Radic. Biol. Med. 79, 217–227 (2015).
Wang, C., Pan, Y., Zhang, Q. Y., Wang, F. M. & Kong, L. D. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS ONE 7, e38285 (2012).
Kim, S. M. et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am. J. Physiol. Renal Physiol. 308, F993–F1003 (2015).
Shahzad, K. et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 87, 74–84 (2015).
Gao, P. et al. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J. Diabetes Res. 2015, 504761 (2015).
Li, J. et al. TLR4 is required for the obesity-induced pancreatic β cell dysfunction. Acta Biochim. Biophys. Sin. 45, 1030–1038 (2013).
Zhou, Y. J., Zhou, H., Li, Y. & Song, Y. L. NOD1 activation induces innate immune responses and insulin resistance in human adipocytes. Diabetes Metab. 38, 538–543 (2012).
Brenner, C. et al. TLR signalling and adapter utilization in primary human in vitro differentiated adipocytes. Scand. J. Immunol. 76, 359–370 (2012).
Kopp, A. et al. Toll-like receptor ligands cause proinflammatory and prodiabetic activation of adipocytes via phosphorylation of extracellular signal-regulated kinase and c-Jun N-terminal kinase but not interferon regulatory factor-3. Endocrinology 151, 1097–1108 (2010).
Liu, P., Li, F., Qiu, M. & He, L. Expression and cellular distribution of TLR4, MyD88, and NF-κB in diabetic renal tubulointerstitial fibrosis, in vitro and in vivo. Diabetes Res. Clin. Pract. 105, 206–216 (2014).
Jin, H. et al. Synergistic effects of leflunomide and benazepril in streptozotocin-induced diabetic nephropathy. Nephron Exp. Nephrol. 126, 148–156 (2014).
Kurihara, T. & Bravo, R. Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J. Biol. Chem. 271, 11603–11607 (1996).
Panee, J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 60, 1–12 (2012).
Roca, H. et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J. Biol. Chem. 284, 34342–34354 (2009).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Gnudi, L. A new chance to beat diabetic kidney disease: innate immunity and MCP-1: a matter of good and bad macrophages? Nephrol. Dial. Transplant. 30, 525–527 (2015).
de Zeeuw, D. et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 3, 687–696 (2015).
Li, J. et al. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 75, 508–518 (2015).
Gao, P. et al. Thioredoxin-interacting protein mediates NALP3 inflammasome activation in podocytes during diabetic nephropathy. Biochim. Biophys. Acta 1843, 2448–2460 (2014).
Solini, A. et al. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J. Pathol. 231, 342–353 (2013).
Boini, K. M. et al. Activation of inflammasomes in podocyte injury of mice on the high fat diet: effects of ASC gene deletion and silencing. Biochim. Biophys. Acta 1843, 836–845 (2014).
Takata, S., Sawa, Y., Uchiyama, T. & Ishikawa, H. Expression of Toll-like receptor 4 in glomerular endothelial cells under diabetic conditions. Acta Histochem. Cytochem. 46, 35–42 (2013).
Acknowledgements
J.W. and H.M. are supported in part by grants from the Japanese Society for the Promotion of Science Grant-in-Aid for Scientific Research (grant numbers 25126716 and 26293218). The authors would like to thank Dr Atsuko Nakatsuka (Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Japan) for valuable discussion and critical editing of the manuscript.
Author information
Authors and Affiliations
Contributions
J.W. researched the data and wrote the article. J.W. and H.M. provided a substantial contribution to discussions of the content and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.W. receives speaker honoraria from Astellas, Boehringer Ingelheim, Novartis, Novo Nordisk, and Tanabe Mitsubishi, and receives grant support from Bayer, Daiichi Sankyo, Kyowa Hakko Kirin, MSD, Novo Nordisk, Otsuka, Torii, Pfizer, Takeda, Taisho Toyama, and Tanabe Mitsubishi. H.M. is a consultant for AbbVie, Astellas and Teijin, receives speaker honoraria from Astellas, Boehringer-Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Takeda, and Tanabe Mitsubishi, and receives grant support from Astellas, Daiichi Sankyo, Dainippon Sumitomo, Kyowa Hakko Kirin, MSD, Novo Nordisk, Takeda, and Tanabe Mitsubishi.
Rights and permissions
About this article
Cite this article
Wada, J., Makino, H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 12, 13–26 (2016). https://doi.org/10.1038/nrneph.2015.175
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.175
This article is cited by
-
NOD1 deficiency ameliorates the progression of diabetic retinopathy by modulating bone marrow–retina crosstalk
Stem Cell Research & Therapy (2024)
-
HMGN1 down-regulation in the diabetic kidney attenuates tubular cells injury and protects against renal inflammation via suppressing MCP-1 and KIM-1 expression through TLR4
Journal of Endocrinological Investigation (2024)
-
Gasdermins and pyroptosis in the kidney
Nature Reviews Nephrology (2023)
-
Long non-coding RNA DLX6-AS1 is the key mediator of glomerular podocyte injury and albuminuria in diabetic nephropathy by targeting the miR-346/GSK-3β signaling pathway
Cell Death & Disease (2023)
-
The effects of fine particulate matter, solid fuel use and greenness on the risks of diabetes in middle-aged and older Chinese
Journal of Exposure Science & Environmental Epidemiology (2023)