Key Points
-
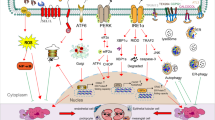
Endoplasmic reticulum (ER) proteostasis is regulated by the adaptive unfolded protein response (UPR) pathway, which determines cell fate and maintains cell structure and function
-
Stress (hypoxia, inflammation and oxidative and glycative stress) disturbs ER proteostasis; cells subjected to long-term or severe ER stress are cleared via the apoptotic UPR pathway
-
In kidney cells, including glomerular cells, tubular cells and interstitial cells, derangement of ER proteostasis leads to development and progression of kidney diseases
-
Optimizing ER proteostasis using pharmacological approaches, such as small-molecule UPR modulators, might prove beneficial to prevent and treat kidney diseases
Abstract
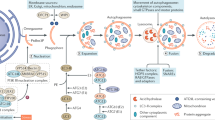
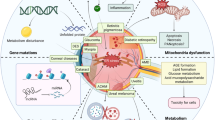
Cells use an exquisite network of mechanisms to maintain the integrity and functionality of their protein components. In the endoplasmic reticulum (ER), these networks of protein homeostasis—referred to as proteostasis—regulate protein synthesis, folding and degradation via the unfolded protein response (UPR) pathway. The UPR pathway has two components: the adaptive UPR pathway, which predominantly maintains the ER function or ER proteostasis, and the apoptotic UPR pathway, which eliminates dysfunctional cells that have been subject to long-term or severe ER stress. Dysregulation of the UPR pathway often occurs in glomerular or tubulointerstitial cells under a pathogenic microenvironment, such as oxidative stress, glycative stress or hypoxia. A defective UPR is highly deleterious to renal cell function and viability and is thereby implicated in the pathophysiology of various kidney diseases. Accumulating evidence provides a link between the UPR pathway and mitochondrial structure and function, indicating the important role of ER proteostasis in the maintenance of mitochondrial homeostasis. Restoration of normal proteostasis, therefore, holds promise in protecting the kidney from pathogenic stresses as well as ageing. This Review is focused on the role of the ER stress and UPR pathway in the maintenance of ER proteostasis, and highlights the involvement of the derangement of ER proteostasis and ER stress in various pathogenic stress signals in the kidney.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Balch, W. E., Morimoto, R. I., Dillin, A. & Kelly, J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008).
Powers, E. T. & Balch, W. E. Diversity in the origins of proteostasis networks—a driver for protein function in evolution. Nat. Rev. Mol. Cell Biol. 14, 237–248 (2013).
Pellegrino, M. W., Nargund, A. M. & Haynes, C. M. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta 1833, 410–416 (2013).
Cook, C., Stetler, C. & Petrucelli, L. Disruption of protein quality control in Parkinson's disease. Cold Spring Harb. Perspect. Med. 2, a009423 (2012).
Selkoe, D. J. Alzheimer's disease. Cold Spring Harb. Perspect. Biol. 3, a004457 (2011).
Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 10, 156–165 (2010).
Burton, G. J., Jauniaux, E. & Charnock-Jones, D. S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 54, 303–312 (2010).
Kimata, Y., Oikawa, D., Shimizu, Y., Ishiwata-Kimata, Y. & Kohno, K. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol. 167, 445–456 (2004).
Pincus, D. et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 (2010).
Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M. & Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 18773–18784 (2005).
Hom, J. R., Gewandter, J. S., Michael, L., Sheu, S. S. & Yoon, Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J. Cell Physiol. 212, 498–508 (2007).
Dickhout, J. G., Carlisle, R. E. & Austin, R. C. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ. Res. 108, 629–642 (2011).
Scheper, W., Nijholt, D. A. & Hoozemans, J. J. The unfolded protein response and proteostasis in Alzheimer disease: preferential activation of autophagy by endoplasmic reticulum stress. Autophagy 7, 910–911 (2011).
Resenberger, U. K. et al. The heat shock response is modulated by and interferes with toxic effects of scrapie prion protein and amyloid β. J. Biol. Chem. 287, 43765–43776 (2012).
Ayala, P. et al. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp. Mol. Pathol. 92, 97–104 (2012).
Park, C. S., Cha, H., Kwon, E. J., Sreenivasaiah, P. K. & Kim, D. H. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 421, 578–584 (2012).
Liu, Y. & Ye, Y. Proteostasis regulation at the endoplasmic reticulum: a new perturbation site for targeted cancer therapy. Cell Res. 21, 867–883 (2011).
Engin, F. & Hotamisligil, G. S. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 12 (Suppl. 2), 108–115 (2010).
Tanaka, T. & Nangaku, M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1α in progression of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 19, 43–50 (2010).
Tanaka, T., Yamaguchi, J., Higashijima, Y. & Nangaku, M. Indoxyl sulfate signals for rapid mRNA stabilization of Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2) and suppresses the expression of hypoxia-inducible genes in experimental CKD and uremia. FASEB J. 27, 4059–4075 (2013).
Gaikwad, A. B., Gupta, J. & Tikoo, K. Epigenetic changes and alteration of Fbn1 and Col3A1 gene expression under hyperglycaemic and hyperinsulinaemic conditions. Biochem. J. 432, 333–341 (2010).
Villeneuve, L. M. & Natarajan, R. Epigenetic mechanisms. Contrib. Nephrol. 170, 57–65 (2011).
Mimura, I. et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol. Cell Biol. 32, 3018–3032 (2012).
Muratsu-Ikeda, S. et al. Downregulation of miR-205 modulates cell susceptibility to oxidative and endoplasmic reticulum stresses in renal tubular cells. PLoS ONE 7, e41462 (2012).
Yang, F. et al. Modulation of the unfolded protein response is the core of microRNA-122-involved sensitivity to chemotherapy in hepatocellular carcinoma. Neoplasia 13, 590–600 (2011).
Nallamshetty, S., Chan, S. Y. & Loscalzo, J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic. Biol. Med. 64, 20–30 (2013).
Queisser, M. A. et al. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 59, 670–678 (2010).
Kumagai, T. et al. Glyoxalase I overexpression ameliorates renal ischemia-reperfusion injury in rats. Am. J. Physiol. Renal Physiol. 296, F912–F921 (2009).
Ikeda, Y. et al. Glyoxalase I retards renal senescence. Am. J. Pathol. 179, 2810–2821 (2011).
Inagi, R. Inhibitors of advanced glycation and endoplasmic reticulum stress. Methods Enzymol. 491, 361–380 (2011).
Piperi, C., Adamopoulos, C., Dalagiorgou, G., Diamanti-Kandarakis, E. & Papavassiliou, A. G. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab. 97, 2231–2242 (2012).
Chen, Y. et al. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am. J. Nephrol. 28, 1014–1022 (2008).
Choudhury, D. & Levi, M. Kidney aging—inevitable or preventable? Nat. Rev. Nephrol. 7, 706–717 (2011).
Vlassara, H. et al. Managing chronic inflammation in the aging diabetic patient with CKD by diet or sevelamer carbonate: a modern paradigm shift. J. Gerontol. A Biol. Sci. Med. Sci. 67, 1410–1416 (2012).
Liu, J. et al. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell. Signal. 26, 110–121 (2013).
Tsakiri, E. N. et al. Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radic. Biol. Med. 65, 1155–1163 (2013).
Nakajima, S. et al. Pleiotropic potential of dehydroxymethylepoxyquinomicin for NF-κB suppression via reactive oxygen species and unfolded protein response. J. Immunol. 190, 6559–6569 (2013).
Hayakawa, K. et al. ER stress depresses NF-κB activation in mesangial cells through preferential induction of C/EBP-β. J. Am. Soc. Nephrol. 21, 73–81 (2010).
Fougeray, S. et al. Metabolic stress promotes renal tubular inflammation by triggering the unfolded protein response. Cell Death Dis. 2, e143 (2011).
Hale, A. N., Ledbetter, D. J., Gawriluk, T. R. & Rucker, E. B. Autophagy: regulation and role in development. Autophagy 9, 951–972 (2013).
Cybulsky, A. V. The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int. 84, 25–33 (2013).
Pallet, N. et al. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy 4, 783–791 (2008).
Kawakami, T. et al. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrol. Dial. Transplant. 24, 2665–2672 (2009).
Yamahara, K. et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J. Am. Soc. Nephrol. 24, 1769–1781 (2013).
Ishihara, M. et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am. J. Physiol. Renal Physiol. 305, F495–F509 (2013).
de Brito, O. M. & Scorrano, L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 29, 2715–2723 (2010).
Vannuvel, K., Renard, P., Raes, M. & Arnould, T. Functional and morphological impact of ER stress on mitochondria. J. Cell. Physiol. 228, 1802–1818 (2013).
Iwasawa, R., Mahul-Mellier, A. L., Datler, C., Pazarentzos, E. & Grimm, S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 30, 556–568 (2011).
de Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008).
Muñoz, J. P. et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 32, 2348–2361 (2013).
Jin, S. M. & Youle, R. J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 125, 795–799 (2012).
Lim, J. H., Lee, H. J., Ho Jung, M. & Song, J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal. 21, 169–177 (2009).
Rieusset, J. Mitochondria and endoplasmic reticulum: mitochondria-endoplasmic reticulum interplay in type 2 diabetes pathophysiology. Int. J. Biochem. Cell Biol. 43, 1257–1262 (2011).
Inagi, R. et al. Preconditioning with endoplasmic reticulum stress ameliorates mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 19, 915–922 (2008).
Chiang, C. K. & Inagi, R. Glomerular diseases: genetic causes and future therapeutics. Nat. Rev. Nephrol. 6, 539–554 (2010).
Cybulsky, A. V. et al. Glomerular epithelial cell injury associated with mutant α-actinin-4. Am. J. Physiol. Renal Physiol. 297, F987–F995 (2009).
He, F. et al. Regulation of CD2-associated protein influences podocyte endoplasmic reticulum stress-mediated apoptosis induced by albumin overload. Gene 484, 18–25 (2011).
Chen, Y. M. et al. Laminin β2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J. Am. Soc. Nephrol. 24, 1223–1233 (2013).
Sieber, J. et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Renal Physiol. 299, F821–F829 (2010).
Hartleben, B. et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 120, 1084–1096 (2010).
Cybulsky, A. V. Membranous nephropathy. Contrib. Nephrol. 169, 107–125 (2011).
Inagi, R. et al. Involvement of endoplasmic reticulum (ER) stress in podocyte injury induced by excessive protein accumulation. Kidney Int. 68, 2639–2650 (2005).
Inagi, R. in An Update on Glomerulopathies–Etiology and Pathogenesis (ed. Prabhakar, S.) 247–266 (InTech, 2011).
Chen, S. et al. Calcium entry via TRPC6 mediates albumin overload-induced endoplasmic reticulum stress and apoptosis in podocytes. Cell Calcium. 50, 523–529 (2011).
Ito, N. et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab. Invest. 91, 1584–1595 (2011).
Inoki, K. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 (2011).
Morse, E. et al. TRB3 is stimulated in diabetic kidneys, regulated by the ER stress marker CHOP, and is a suppressor of podocyte MCP 1. Am. J. Physiol. Renal Physiol. 299, F965–F972 (2010).
Ohse, T. et al. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 70, 1447–1455 (2006).
Lindenmeyer, M. T. et al. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J. Am. Soc. Nephrol. 19, 2225–2236 (2008).
Kawakami, T. et al. Indoxyl sulfate inhibits proliferation of human proximal tubular cells via endoplasmic reticulum stress. Am. J. Physiol. Renal Physiol. 299, F568–F576 (2010).
Khan, M. A. et al. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 27, 2946–2956 (2013).
Bando, Y. et al. ORP150/HSP12A protects renal tubular epithelium from ischemia-induced cell death. FASEB J. 18, 1401–1403 (2004).
Yu, W. et al. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J. Transl. Med. 11, 24 (2013).
Takeda, N. et al. Altered unfolded protein response is implicated in the age-related exacerbation of proteinuria-induced proximal tubular cell damage. Am. J. Pathol. 183, 774–785 (2013).
Ren, S. & Duffield, J. S. Pericytes in kidney fibrosis. Curr. Opin. Nephrol. Hypertens. 22, 471–480 (2013).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Chiang, C. K. et al. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol. Med. 17, 1295–1305 (2011).
Dihazi, H. et al. Secretion of ERP57 is important for extracellular matrix accumulation and progression of renal fibrosis, and is an early sign of disease onset. J. Cell Sci. 126, 3649–3663 (2013).
Souma, T. et al. Plasticity of renal erythropoietin-producing cells governs fibrosis. J. Am. Soc. Nephrol. 24, 1599–1616 (2013).
La Ferla, K., Reimann, C., Jelkmann, W. & Hellwig-Bürgel, T. Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-κB. FASEB J. 16, 1811–1813 (2002).
Chiang, C. K., Nangaku, M., Tanaka, T., Iwawaki, T. & Inagi, R. Endoplasmic reticulum stress signal impairs erythropoietin production: a role for ATF4. Am. J. Physiol. Cell Physiol. 304, C342–C353 (2013).
Stetler, R. A. et al. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 92, 184–211 (2010).
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011).
Kim, J. Y. et al. In vivo activating transcription factor 3 silencing ameliorates the AMPK compensatory effects for ER stress-mediated β cell dysfunction during the progression of type 2 diabetes. Cell. Signal. 25, 2348–2361 (2013).
Taylor, R. C. & Dillin, A. Aging as an event of proteostasis collapse. Cold Spring Harb. Perspect. Biol. 3, a004440 (2011).
Cao, S. S. & Kaufman, R. J. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin. Ther. Targets 17, 437–448 (2013).
Liu, X. L. et al. Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J. Am. Soc. Nephrol. 15, 1731–1738 (2004).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Han, J. et al. ER stress induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Finley, D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 (2009).
Husnjak, K. & Dikic, I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 81, 291–322 (2012).
Tyedmers, J., Mogk, A. & Bukau, B. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11, 777–788 (2010).
Chen, B., Retzlaff, M., Roos, T. & Frydman, J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 3, a004374 (2011).
Lindquist, S. L. & Kelly, J. W. Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb. Perspect. Biol. 3, a004507 (2011).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009).
Luo, Z. F. et al. Effects of 4 phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicol. Appl. Pharmacol. 246, 49–57 (2010).
Albuquerque, J. A., Lamers, M. L., Castiblanco-Valencia, M. M., Dos Santos, M. & Isaac, L. Chemical chaperones curcumin and 4 phenylbutyric acid improve secretion of mutant factor H R127H by fibroblasts from a factor H deficient patient. J. Immunol. 189, 3242–3248 (2012).
Wang, J. Q. et al. Phenylbutyric acid protects against carbon tetrachloride-induced hepatic fibrogenesis in mice. Toxicol. Appl. Pharmacol. 266, 307–316 (2013).
Omura, T. et al. Sodium tauroursodeoxycholate prevents paraquat-induced cell death by suppressing endoplasmic reticulum stress responses in human lung epithelial A549 cells. Biochem. Biophys. Res. Commun. 432, 689–694 (2013).
Kudo, T. et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 15, 364–375 (2008).
Prachasilchai, W. et al. The protective effect of a newly developed molecular chaperone-inducer against mouse ischemic acute kidney injury. J. Pharmacol. Sci. 109, 311–314 (2009).
Benjamin, E. R. et al. The pharmacological chaperone 1 deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. J. Inherit. Metab. Dis. 32, 424–440 (2009).
Germain, D. P. et al. Safety and pharmacodynamic effects of a pharmacological chaperone on α galactosidase A activity and globotriaosylceramide clearance in Fabry disease: report from two phase 2 clinical studies. Orphanet J. Rare Dis. 7, 91 (2012).
Giugliani, R. et al. A Phase 2 study of migalastat hydrochloride in females with Fabry disease: selection of population, safety and pharmacodynamic effects. Mol. Genet. Metab. 109, 86–92 (2013).
Moreno, J. A. et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 5, 206ra138 (2013).
Fang, L. et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS ONE 8, e60546 (2013).
Pallet, N. et al. Cyclosporine-induced endoplasmic reticulum stress triggers tubular phenotypic changes and death. Am. J. Transplant. 8, 2283–2296 (2008).
Bouvier, N. et al. Cyclosporine triggers endoplasmic reticulum stress in endothelial cells: a role for endothelial phenotypic changes and death. Am. J. Physiol. Renal Physiol. 296, F160–F169 (2009).
Holmes, B., Brogden, R. N., Heel, R. C., Speight, T. M. & Avery, G. S. Guanabenz. A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs 26, 212–229 (1983).
Tsaytler, P., Harding, H. P., Ron, D. & Bertolotti, A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332, 91–94 (2011).
Boots, A. W., Haenen, G. R. & Bast, A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 585, 325–337 (2008).
Liu, C. M., Zheng, G. H., Ming, Q. L., Sun, J. M. & Cheng, C. Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway. Free Radic. Res. 47, 192–201 (2013).
Shoskes, D. A. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation 66, 147–152 (1998).
Meyer-Schwesinger, C. et al. A new role for the neuronal ubiquitin C terminal hydrolase L1 (UCH L1) in podocyte process formation and podocyte injury in human glomerulopathies. J. Pathol. 217, 452–464 (2009).
Duensing, S. & Duensing, A. Bortezomib: killing two birds with one stone in gastrointestinal stromal tumors. Oncotarget 1, 6–8 (2010).
Zangari, M., Terpos, E., Zhan, F. & Tricot, G. Impact of bortezomib on bone health in myeloma: a review of current evidence. Cancer Treat. Rev. 38, 968–980 (2012).
Sureshkumar, K. K. et al. Proteasome inhibition with bortezomib: an effective therapy for severe antibody mediated rejection after renal transplantation. Clin. Nephrol. 77, 246–253 (2012).
Tzvetanov, I. et al. The use of bortezomib as a rescue treatment for acute antibody-mediated rejection: report of three cases and review of literature. Transplant. Proc. 44, 2971–2975 (2012).
Danilov, S. M. et al. Angiotensin I converting enzyme Gln1069Arg mutation impairs trafficking to the cell surface resulting in selective denaturation of the C domain. PLoS ONE 5, e10438 (2010).
Zang, Y. et al. The next generation proteasome inhibitors carfilzomib and oprozomib activate prosurvival autophagy via induction of the unfolded protein response and ATF4. Autophagy 8, 1873–1874 (2012).
Li, Y. et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 25, 1664–1679 (2011).
Jung, T. W., Lee, K. T., Lee, M. W. & Ka, K. H. SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem. Biophys. Res. Commun. 422, 229–232 (2012).
Wang, F. M., Chen, Y. J. & Ouyang, H. J. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem. J. 433, 245–252 (2011).
Acknowledgements
The authors' research work is supported by Grants-in-Aid for Scientific Research No. 25461207 (to R.I.) and 24390213 (to M.N.) from the Japan Society for the Promotion of Science, by the Japanese Association of Dialysis Physicians (JADP Grant 2012-05 to R.I.) and by Kyowa Hakko Kirin Pharmaceutical Company in Japan (to R.I.).
Author information
Authors and Affiliations
Contributions
R.I. and Y.I. researched the data for the article and wrote the manuscript. All authors contributed to the discussion of the article's content and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Inagi, R., Ishimoto, Y. & Nangaku, M. Proteostasis in endoplasmic reticulum—new mechanisms in kidney disease. Nat Rev Nephrol 10, 369–378 (2014). https://doi.org/10.1038/nrneph.2014.67
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.67
This article is cited by
-
Endoplasmic reticulum stress contributes to cisplatin-induced chronic kidney disease via the PERK–PKCδ pathway
Cellular and Molecular Life Sciences (2022)
-
Downregulation of XBP1 protects kidney against ischemia-reperfusion injury via suppressing HRD1-mediated NRF2 ubiquitylation
Cell Death Discovery (2021)
-
Mitochondrial quality control in kidney injury and repair
Nature Reviews Nephrology (2021)
-
Reciprocal regulation between ER stress and autophagy in renal tubular fibrosis and apoptosis
Cell Death & Disease (2021)
-
Tubular Mas receptor mediates lipid-induced kidney injury
Cell Death & Disease (2021)