Key Points
-
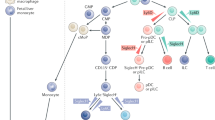
Dendritic cells (DCs) and macrophages are distinct cell types, but demonstrate similarities in terms of ontogeny, phenotype, and function
-
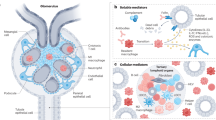
Both of these cell types are present within the renal interstitium and are critical to homeostatic regulation of the kidney environment
-
The numbers of DCs and macrophages in the kidney increase following renal injury
-
A variety of DC and macrophage subtypes exist, each with distinct phenotypes and activities, and many can be identified based on panels of cellular markers
-
The manifestation of glomerular or tubular kidney disease, as well as disease outcome in preclinical models, is determined by the subtype of DC and/or macrophage involved
-
A number of pharmacological or genetic approaches are available to deplete or eliminate DCs or macrophages in preclinical models, but the effects of such interventions are not renal-specific
Abstract
Renal dendritic cells (DCs) and macrophages represent a constitutive, extensive and contiguous network of innate immune cells that provide sentinel and immune-intelligence activity; they induce and regulate inflammatory responses to freely filtered antigenic material and protect the kidney from infection. Tissue-resident or infiltrating DCs and macrophages are key factors in the initiation and propagation of renal disease, as well as essential contributors to subsequent tissue regeneration, regardless of the aetiological and pathogenetic mechanisms. The identification, and functional and phenotypic distinction of these cell types is complex and incompletely understood, and the same is true of their interplay and relationships with effector and regulatory cells of the adaptive immune system. In this Review, we discuss the common and distinct characteristics of DCs and macrophages, as well as key advances that have identified the renal-specific functions of these important phagocytic, antigen-presenting cells, and their roles in potentiating or mitigating intrinsic kidney disease. We also identify remaining issues that are of priority for further investigation, and highlight the prospects for translational and therapeutic application of the knowledge acquired.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Geissmann, F. The origin of dendritic cells. Nat. Immunol. 8, 558–560 (2007).
Shortman, K. & Liu, Y. J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161 (2002).
Nelson, P. J. et al. The renal mononuclear phagocytic system. J. Am. Soc. Nephrol. 23, 194–203 (2012).
Lyman, S. D. et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75, 1157–1167 (1993).
Wu, L. & Liu, Y. J. Development of dendritic-cell lineages. Immunity 26, 741–750 (2007).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7, 19–30 (2007).
Lin, S. L., Castano, A. P., Nowlin, B. T., Lupher, M. L. Jr & Duffield, J. S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743 (2009).
Miller, J. C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Hume, D. A. Plenary perspective: the complexity of constitutive and inducible gene expression in mononuclear phagocytes. J. Leukoc. Biol. 92, 433–444 (2012).
Hume, D. A. Macrophages as APC and the dendritic cell myth. J. Immunol. 181, 5829–5835 (2008).
Hume, D. A., Mabbott, N., Raza, S. & Freeman, T. C. Can DCs be distinguished from macrophages by molecular signatures? Nat. Immunol. 14, 187–189 (2013).
Ferenbach, D. & Hughes, J. Macrophages and dendritic cells: what is the difference? Kidney Int. 74, 5–7 (2008).
Kawakami, T. et al. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J. Immunol. 191, 3358–3372 (2013).
Cailhier, J. F. et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J. Immunol. 174, 2336–2342 (2005).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Steinman, R. M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9, 271–296 (1991).
Naik, S. H. Demystifying the development of dendritic cell subtypes, a little. Immunol. Cell Biol. 86, 439–452 (2008).
Morelli, A. E. & Thomson, A. W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 7, 610–621 (2007).
Steinman, R. M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Liu, K. & Nussenzweig, M. C. Origin and development of dendritic cells. Immunol. Rev. 234, 45–54 (2010).
Mellman, I. & Steinman, R. M. Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258 (2001).
Kapsenberg, M. L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3, 984–993 (2003).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Diebold, S. S. Determination of T-cell fate by dendritic cells. Immunol. Cell Biol. 86, 389–397 (2008).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Hume, D. A. & MacDonald, K. P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820 (2012).
Alikhan, M. A. et al. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am. J. Pathol. 179, 1243–1256 (2011).
Rae, F. et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev. Biol. 308, 232–246 (2007).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Sieweke, M. H. & Allen, J. E. Beyond stem cells: self-renewal of differentiated macrophages. Science 342, 1242974 (2014).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Rees, A. J. Monocyte and macrophage biology: an overview. Semin. Nephrol. 30, 216–233 (2010).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Ascon, D. B. et al. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J. Immunol. 177, 3380–3387 (2006).
Paust, H. J. et al. Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int. 80, 154–164 (2011).
Wu, H. et al. Depletion of γδ T cells exacerbates murine adriamycin nephropathy. J. Am. Soc. Nephrol. 18, 1180–1189 (2007).
Steinman, R. M. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142–1162 (1973).
Steinman, R. M. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J. Exp. Med. 139, 380–397 (1974).
Steinman, R. M., Lustig, D. S. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. 3. Functional properties in vivo. J. Exp. Med. 139, 1431–1445 (1974).
Schreiner, G. F., Kiely, J. M., Cotran, R. S. & Unanue, E. R. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J. Clin. Invest. 68, 920–931 (1981).
Schreiner, G. F., Cotran, R. S. & Unanue, E. R. Modulation of Ia and leukocyte common antigen expression in rat glomeruli during the course of glomerulonephritis and aminonucleoside nephrosis. Lab. Invest. 51, 524–533 (1984).
Schreiner, G. F. & Cotran, R. S. Localization of an Ia-bearing glomerular cell in the mesangium. J. Cell Biol. 94, 483–488 (1982).
Gieseler, R. et al. Enrichment and characterization of dendritic cells from rat renal mesangium. Scand. J. Immunol. 46, 587–596 (1997).
Hart, D. N. & Fabre, J. W. Major histocompatibility complex antigens in rat kidney, ureter, and bladder. Localization with monoclonal antibodies and demonstration of Ia-positive dendritic cells. Transplantation 31, 318–325 (1981).
Hart, D. N. et al. Localization of HLA-ABC and DR antigens in human kidney. Transplantation 31, 428–433 (1981).
Kaissling, B., Hegyi, I., Loffing, J. & Le Hir, M. Morphology of interstitial cells in the healthy kidney. Anat. Embryol. (Berl.) 193, 303–318 (1996).
Pajak, B. et al. Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualisation of dendritic cells in peripheral organs. J. Clin. Pathol. 53, 518–524 (2000).
Kruger, T. et al. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J. Am. Soc. Nephrol. 15, 613–621 (2004).
Ginhoux, F. et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 (2009).
Coates, P. T. et al. In vivo-mobilized kidney dendritic cells are functionally immature, subvert alloreactive T-cell responses, and prolong organ allograft survival. Transplantation 77, 1080–1089 (2004).
Morelli, A. E. et al. Growth factor-induced mobilization of dendritic cells in kidney and liver of rhesus macaques: implications for transplantation. Transplantation 83, 656–662 (2007).
Coates, P. T. et al. CCR and CC chemokine expression in relation to Flt3 ligand-induced renal dendritic cell mobilization. Kidney Int. 66, 1907–1917 (2004).
Schraml, B. U. et al. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 154, 843–858 (2013).
Li, L. et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 74, 1526–1537 (2008).
MacDonald, K. P. et al. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J. Immunol. 175, 1399–1405 (2005).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Sunderkotter, C. et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417 (2004).
Lee, S. et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 22, 317–326 (2011).
Anders, H. J. & Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 80, 915–925 (2011).
Lin, S. L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl Acad. Sci. USA 107, 4194–4199 (2010).
Soos, T. J. et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70, 591–596 (2006).
Sasmono, R. T. et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101, 1155–1163 (2003).
Hume, D. A. & Gordon, S. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J. Exp. Med. 157, 1704–1709 (1983).
Masaki, T., Chow, F., Nikolic-Paterson, D. J., Atkins, R. C. & Tesch, G. H. Heterogeneity of antigen expression explains controversy over glomerular macrophage accumulation in mouse glomerulonephritis. Nephrol. Dial. Transplant. 18, 178–181 (2003).
van Rooijen, N. Liposomes as an in vivo tool to study and manipulate macrophage function. Res. Immunol. 143, 177–178 (1992).
van Rooijen, N. Liposome-mediated elimination of macrophages. Res. Immunol. 143, 215–219 (1992).
van Rooijen, N. & Sanders, A. Elimination, blocking, and activation of macrophages: three of a kind? J. Leukoc. Biol. 62, 702–709 (1997).
van Rooijen, N., Sanders, A. & van den Berg, T. K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods 193, 93–99 (1996).
van Rooijen, N. & van Nieuwmegen, R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 238, 355–358 (1984).
Lu, L. et al. Depletion of macrophages and dendritic cells in ischemic acute kidney injury. Am. J. Nephrol. 35, 181–190 (2012).
Ferenbach, D. A. et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 82, 928–933 (2012).
Tittel, A. P. et al. Kidney dendritic cells induce innate immunity against bacterial pyelonephritis. J. Am. Soc. Nephrol. 22, 1435–1441 (2011).
Vinuesa, E. et al. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J. Pathol. 214, 104–113 (2008).
Kulkarni, O. P. et al. Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J. Am. Soc. Nephrol. 25, 978–989 (2014).
Kaissling, B., Lehir, M. & Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 1832, 931–939 (2013).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Sung, S. A., Jo, S. K., Cho, W. Y., Won, N. H. & Kim, H. K. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron Exp. Nephrol. 105, e1–e9 (2007).
Kitamoto, K. et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J. Pharmacol. Sci. 111, 285–292 (2009).
Henderson, N. C. et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 172, 288–298 (2008).
Machida, Y. et al. Renal fibrosis in murine obstructive nephropathy is attenuated by depletion of monocyte lineage, not dendritic cells. J. Pharmacol. Sci. 114, 464–473 (2010).
Snelgrove, S. L. et al. Renal dendritic cells adopt a pro-inflammatory phenotype in obstructive uropathy to activate T cells but do not directly contribute to fibrosis. Am. J. Pathol. 180, 91–103 (2012).
Lech, M. et al. Macrophage phenotype controls long-term AKI outcomes—kidney regeneration versus atrophy. J. Am. Soc. Nephrol. 25, 292–304 (2014).
Huen, S. C., Moeckel, G. W. & Cantley, L. G. Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am. J. Physiol. Renal Physiol. 305, F477–F484 (2013).
Lukacs-Kornek, V. et al. The kidney-renal lymph node-system contributes to cross-tolerance against innocuous circulating antigen. J. Immunol. 180, 706–715 (2008).
Gottschalk, C. et al. Batf3-dependent dendritic cells in the renal lymph node induce tolerance against circulating antigens. J. Am. Soc. Nephrol. 24, 543–549 (2013).
Macconi, D. et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J. Am. Soc. Nephrol. 20, 123–130 (2009).
Dong, X. et al. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 68, 1096–1108 (2005).
Roake, J. A. et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 181, 2237–2247 (1995).
Kurts, C., Heymann, F., Lukacs-Kornek, V., Boor, P. & Floege, J. Role of T cells and dendritic cells in glomerular immunopathology. Semin. Immunopathol. 29, 317–335 (2007).
Assmann, K. J., Tangelder, M. M., Lange, W. P., Schrijver, G. & Koene, R. A. Anti-GBM nephritis in the mouse: severe proteinuria in the heterologous phase. Virchows Arch. A Pathol. Anat. Histopathol. 406, 285–299 (1985).
Hochheiser, K. et al. Kidney dendritic cells become pathogenic during crescentic glomerulonephritis with proteinuria. J. Am. Soc. Nephrol. 22, 306–316 (2011).
Lichtnekert, J. et al. Anti-GBM glomerulonephritis involves IL-1 but is independent of NLRP3/ASC inflammasome-mediated activation of caspase-1. PLoS ONE 6, e26778 (2011).
Scholz, J. et al. Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J. Am. Soc. Nephrol. 19, 527–537 (2008).
Kitching, A. R., Tipping, P. G., Huang, X. R., Mutch, D. A. & Holdsworth, S. R. Interleukin-4 and interleukin-10 attenuate established crescentic glomerulonephritis in mice. Kidney Int. 52, 52–59 (1997).
Kitching, A. R., Tipping, P. G., Timoshanko, J. R. & Holdsworth, S. R. Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int. 57, 518–525 (2000).
Witsch, E. J. et al. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur. J. Immunol. 32, 2680–2686 (2002).
Duffield, J. S. et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am. J. Pathol. 167, 1207–1219 (2005).
Lloyd, C. M., Dorf, M. E., Proudfoot, A., Salant, D. J. & Gutierrez-Ramos, J. C. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J. Leukoc. Biol. 62, 676–680 (1997).
Wada, T. et al. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1). FASEB J. 10, 1418–1425 (1996).
Yamamoto, T. & Wilson, C. B. Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int. 32, 514–525 (1987).
Ikezumi, Y., Hurst, L. A., Masaki, T., Atkins, R. C. & Nikolic-Paterson, D. J. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 63, 83–95 (2003).
Lan, H. Y., Nikolic-Paterson, D. J., Mu, W. & Atkins, R. C. Local macrophage proliferation in the progression of glomerular and tubulointerstitial injury in rat anti-GBM glomerulonephritis. Kidney Int. 48, 753–760 (1995).
Han, Y., Ma, F. Y., Tesch, G. H., Manthey, C. L. & Nikolic-Paterson, D. J. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab. Invest. 91, 978–991 (2011).
Odobasic, D. et al. Glomerular expression of CD80 and CD86 is required for leukocyte accumulation and injury in crescentic glomerulonephritis. J. Am. Soc. Nephrol. 16, 2012–2022 (2005).
Heymann, F. et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Invest. 119, 1286–1297 (2009).
Schiffer, L. et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J. Immunol. 180, 1938–1947 (2008).
Ishikawa, S. et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J. Exp. Med. 193, 1393–1402 (2001).
Castellano, G. et al. Infiltrating dendritic cells contribute to local synthesis of C1q in murine and human lupus nephritis. Mol. Immunol. 47, 2129–2137 (2010).
Kikawada, E., Lenda, D. M. & Kelley, V. R. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-γ expression, and diminishes systemic pathology. J. Immunol. 170, 3915–3925 (2003).
Kulkarni, O. et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 18, 2350–2358 (2007).
Perez de Lema, G. et al. Chemokine receptor Ccr2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J. Am. Soc. Nephrol. 16, 3592–3601 (2005).
Wan, S., Xia, C. & Morel, L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J. Immunol. 178, 271–279 (2007).
Wan, S., Zhou, Z., Duan, B. & Morel, L. Direct B cell stimulation by dendritic cells in a mouse model of lupus. Arthritis Rheum. 58, 1741–1750 (2008).
Bagavant, H., Thompson, C., Ohno, K., Setiady, Y. & Tung, K. S. Differential effect of neonatal thymectomy on systemic and organ-specific autoimmune disease. Int. Immunol. 14, 1397–1406 (2002).
Bagavant, H., Deshmukh, U. S., Wang, H., Ly, T. & Fu, S. M. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J. Immunol. 177, 8258–8265 (2006).
Schiffer, L. et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J. Immunol. 171, 489–497 (2003).
Iwata, Y. et al. p38 Mitogen-activated protein kinase contributes to autoimmune renal injury in MRL-Fas lpr mice. J. Am. Soc. Nephrol. 14, 57–67 (2003).
Iwata, Y. et al. Dendritic cells contribute to autoimmune kidney injury in MRL-Faslpr mice. J. Rheumatol. 36, 306–314 (2009).
Baccala, R. et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc. Natl Acad. Sci. USA 110, 2940–2945 (2012).
Kinjoh, K., Kyogoku, M. & Good, R. A. Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc. Natl Acad. Sci. USA 90, 3413–3417 (1993).
Saiga, K. et al. NK026680, a novel suppressant of dendritic cell function, prevents the development of rapidly progressive glomerulonephritis and perinuclear antineutrophil cytoplasmic antibody in SCG/Kj mice. Arthritis Rheum. 54, 3707–3715 (2006).
Zhang, Y. et al. Immune complex enhances tolerogenecity of immature dendritic cells via FcγRIIb and promotes FcγRIIb-overexpressing dendritic cells to attenuate lupus. Eur. J. Immunol. 41, 1154–1164 (2011).
McGaha, T. L., Karlsson, M. C. & Ravetch, J. V. FcγRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J. Immunol. 180, 5670–5679 (2008).
Rodriguez, W. et al. C-reactive protein-mediated suppression of nephrotoxic nephritis: role of macrophages, complement, and Fcγ receptors. J. Immunol. 178, 530–538 (2007).
Zhang, Y. et al. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through Fc γ RIIb-dependent PGE2 production. J. Immunol. 182, 554–562 (2009).
Muhlfeld, A. S. et al. Deletion of the Fcγ receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis. Am. J. Pathol. 163, 1127–1136 (2003).
Smith, J. et al. A spleen tyrosine kinase inhibitor reduces the severity of established glomerulonephritis. J. Am. Soc. Nephrol. 21, 231–236 (2010).
Tadagavadi, R. K. & Reeves, W. B. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J. Am. Soc. Nephrol. 21, 53–63 (2009).
Li, L. & Okusa, M. D. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin. Nephrol 30, 268–277 (2010).
Jo, S. K., Sung, S. A., Cho, W. Y., Go, K. J. & Kim, H. K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transplant. 21, 1231–1239 (2006).
Day, Y. J., Huang, L., Ye, H., Linden, J. & Okusa, M. D. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am. J. Physiol. Renal Physiol. 288, F722–F731 (2005).
Ko, G. J., Boo, C. S., Jo, S. K., Cho, W. Y. & Kim, H. K. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 23, 842–852 (2008).
Zhang, M. Z. et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Invest. 122, 4519–4532 (2012).
Kim, M. G. et al. Depletion of kidney CD11c+ F4/80+ cells impairs the recovery process in ischaemia/reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 25, 2908–2921 (2010).
Tadagavadi, R. K. & Reeves, W. B. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J. Immunol. 185, 4904–4911 (2010).
Kinsey, G. R., Huang, L., Vergis, A. L., Li, L. & Okusa, M. D. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 77, 771–780 (2010).
Kinsey, G. R. et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 20, 1744–1753 (2009).
Gandolfo, M. T. et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 76, 717–729 (2009).
Kim, M. G. et al. IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J. Am. Soc. Nephrol. 24, 1529–1536 (2013).
Cho, W. Y. et al. The role of Tregs and CD11c+ macrophages/dendritic cells in ischemic preconditioning of the kidney. Kidney Int. 78, 981–992 (2010).
Cantaluppi, V. et al. Rationale of mesenchymal stem cell therapy in kidney injury. Am. J. Kidney Dis. 61, 300–309 (2013).
Kim, M. G. et al. CD11c+ cells partially mediate the renoprotective effect induced by bone-marrow-derived mesenchymal stem cells. PLoS ONE 8, e72544 (2013).
Menke, J. et al. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J. Clin. Invest. 119, 2330–2342 (2009).
Wang, Y., Tay, Y. C. & Harris, D. C. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int. 66, 655–662 (2004).
Dong, X. et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 71, 619–628 (2007).
Lassen, S. et al. Ischemia reperfusion induces IFN regulatory factor 4 in renal dendritic cells, which suppresses postischemic inflammation and prevents acute renal failure. J. Immunol. 185, 1976–1983 (2009).
Lech, M. et al. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J. Immunol. 183, 4109–4118 (2009).
Garlanda, C. et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc. Natl Acad. Sci. USA 101, 3522–3526 (2004).
Noris, M. et al. The Toll-IL-1R member Tir8/SIGIRR negatively regulates adaptive immunity against kidney grafts. J. Immunol. 183, 4249–4260 (2009).
Dong, X., Bachman, L. A., Miller, M. N., Nath, K. A. & Griffin, M. D. Dendritic cells facilitate accumulation of IL-17 T cells in the kidney following acute renal obstruction. Kidney Int. 74, 1294–1309 (2008).
Kitagawa, K. et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am. J. Pathol. 165, 237–246 (2004).
Chadban, S. J., Wu, H. & Hughes, J. Macrophages and kidney transplantation. Semin. Nephrol. 30, 278–289 (2010).
Penfield, J. G. et al. Transplant surgery injury recruits recipient MHC class II-positive leukocytes into the kidney. Kidney Int. 56, 1759–1769 (1999).
Penfield, J. G. et al. Syngeneic renal transplantation increases the number of renal dendritic cells in the rat. Transpl. Immunol. 7, 197–200 (1999).
Le Meur, Y., Jose, M. D., Mu, W., Atkins, R. C. & Chadban, S. J. Macrophage colony-stimulating factor expression and macrophage accumulation in renal allograft rejection. Transplantation 73, 1318–1324 (2002).
Milton, A. D., Spencer, S. C. & Fabre, J. W. The effects of cyclosporine on the induction of donor class I and class II MHC antigens in heart and kidney allografts in the rat. Transplantation 42, 337–347 (1986).
Ahn, K. O. et al. Influence of angiotensin II on expression of toll-like receptor 2 and maturation of dendritic cells in chronic cyclosporine nephropathy. Transplantation 83, 938–947 (2007).
Lim, S. W. et al. Cyclosporine-induced renal injury induces toll-like receptor and maturation of dendritic cells. Transplantation 80, 691–699 (2005).
Ozaki, K. S. et al. The loss of renal dendritic cells and activation of host adaptive immunity are long-term effects of ischemia/reperfusion injury following syngeneic kidney transplantation. Kidney Int. 81, 1015–1025 (2012).
Jose, M. D., Ikezumi, Y., van Rooijen, N., Atkins, R. C. & Chadban, S. J. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation 76, 1015–1022 (2003).
Qi, F. et al. Depletion of cells of monocyte lineage prevents loss of renal microvasculature in murine kidney transplantation. Transplantation 86, 1267–1274 (2008).
Jose, M. D., Le Meur, Y., Atkins, R. C. & Chadban, S. J. Blockade of macrophage colony-stimulating factor reduces macrophage proliferation and accumulation in renal allograft rejection. Am. J. Transplant. 3, 294–300 (2003).
Ma, F. Y., Woodman, N., Mulley, W. R., Kanellis, J. & Nikolic-Paterson, D. J. Macrophages contribute to cellular but not humoral mechanisms of acute rejection in rat renal allografts. Transplantation 96, 949–957 (2013).
Shimizu, A., Yamada, K., Sachs, D. H. & Colvin, R. B. Mechanisms of chronic renal allograft rejection. II. Progressive allograft glomerulopathy in miniature swine. Lab. Invest. 82, 673–686 (2002).
Azuma, H., Nadeau, K. C., Ishibashi, M. & Tilney, N. L. Prevention of functional, structural, and molecular changes of chronic rejection of rat renal allografts by a specific macrophage inhibitor. Transplantation 60, 1577–1582 (1995).
Yang, J. et al. Targeting of macrophage activity by adenovirus-mediated intragraft overexpression of TNFRp55-Ig, IL-12p40, and vIL-10 ameliorates adenovirus-mediated chronic graft injury, whereas stimulation of macrophages by overexpression of IFN-γ accelerates chronic graft injury in a rat renal allograft model. J. Am. Soc. Nephrol. 14, 214–225 (2003).
Woltman, A. M. et al. Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int. 71, 1001–1008 (2007).
Mayer, V. et al. Expression of the chemokine receptor CCR1 in human renal allografts. Nephrol. Dial. Transplant. 22, 1720–1729 (2007).
Fahim, T. et al. The cellular lesion of humoral rejection: predominant recruitment of monocytes to peritubular and glomerular capillaries. Am. J. Transplant. 7, 385–393 (2007).
Sund, S., Reisaeter, A. V., Scott, H., Mollnes, T. E. & Hovig, T. Glomerular monocyte/macrophage influx correlates strongly with complement activation in 1-week protocol kidney allograft biopsies. Clin. Nephrol. 62, 121–130 (2004).
Grimm, P. C. et al. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J. Am. Soc. Nephrol. 10, 1582–1589 (1999).
Pilmore, H. L., Painter, D. M., Bishop, G. A., McCaughan, G. W. & Eris, J. M. Early up-regulation of macrophages and myofibroblasts: a new marker for development of chronic renal allograft rejection. Transplantation 69, 2658–2662 (2000).
Bunnag, S. et al. Molecular correlates of renal function in kidney transplant biopsies. J. Am. Soc. Nephrol. 20, 1149–1160 (2009).
Scian, M. J. et al. Gene expression changes are associated with loss of kidney graft function and interstitial fibrosis and tubular atrophy: diagnosis versus prediction. Transplantation 91, 657–665 (2011).
Mengel, M. et al. The molecular phenotype of 6-week protocol biopsies from human renal allografts: reflections of prior injury but not future course. Am. J. Transplant. 11, 708–718 (2011).
Papadimitriou, J. C. et al. Glomerular inflammation in renal allografts biopsies after the first year: cell types and relationship with antibody-mediated rejection and graft outcome. Transplantation 90, 1478–1485 (2010).
Ozdemir, B. H., Demirhan, B. & Gungen, Y. The presence and prognostic importance of glomerular macrophage infiltration in renal allografts. Nephron 90, 442–446 (2002).
Hoffmann, U. et al. Impact of chemokine receptor CX3CR1 in human renal allograft rejection. Transpl. Immunol. 23, 204–208 (2010).
Copin, M. C. et al. Diagnostic and predictive value of an immunohistochemical profile in asymptomatic acute rejection of renal allografts. Transpl. Immunol. 3, 229–239 (1995).
Yoshimoto, K. et al. CD68 and MCP-1/CCR2 expression of initial biopsies reflect the outcomes of membranous nephropathy. Nephron Clin. Pract. 98, c25–c34 (2004).
Eardley, K. S. et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 69, 1189–1197 (2006).
Eardley, K. S. et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 74, 495–504 (2008).
Cuzic, S., Ritz, E. & Waldherr, R. Dendritic cells in glomerulonephritis. Virchows Arch. B Cell Pathol. Incl Mol. Pathol. 62, 357–363 (1992).
De Palma, G. et al. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 79, 1228–1235 (2011).
Segerer, S. et al. Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int. 74, 37–46 (2008).
Noessner, E., Lindenmeyer, M., Nelson, P. J. & Segerer, S. Dendritic cells in human renal inflammation—Part II. Nephron Exp. Nephrol. 119, e91–e98 (2011).
Kassianos, A. J. et al. Increased tubulointerstitial recruitment of human CD141hi CLEC9A+ and CD1c+ myeloid dendritic cell subsets in renal fibrosis and chronic kidney disease. Am. J. Physiol. Renal Physiol. 305, F1391–F1401 (2013).
Tucci, M., Ciavarella, S., Strippoli, S., Dammacco, F. & Silvestris, F. Oversecretion of cytokines and chemokines in lupus nephritis is regulated by intraparenchymal dendritic cells: a review. Ann. N. Y Acad. Sci. 1173, 449–457 (2009).
Tucci, M. et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 58, 251–262 (2008).
Loverre, A. et al. Ischemia-reperfusion injury-induced abnormal dendritic cell traffic in the transplanted kidney with delayed graft function. Kidney Int. 72, 994–1003 (2007).
Wilde, B. et al. Dendritic cells in renal biopsies of patients with ANCA-associated vasculitis. Nephrol. Dial. Transplant. 24, 2151–2156 (2009).
Steinmetz, O. M. et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 74, 448–457 (2008).
Kerjaschki, D. et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 15, 603–612 (2004).
Loike, J. D. et al. CD11c/CD18 on neutrophils recognizes a domain at the N. terminus of the A α chain of fibrinogen. Proc. Natl Acad. Sci. USA 88, 1044–1048 (1991).
Fancke, B., Suter, M., Hochrein, H. & O'Keeffe, M. M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood 111, 150–159 (2008).
Fleetwood, A. J., Lawrence, T., Hamilton, J. A. & Cook, A. D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 178, 5245–5252 (2007).
Hume, D. A. Macrophages as APC and the dendritic cell myth. J. Immunol. 181, 5829–5835 (2008).
Desmedt, M., Rottiers, P., Dooms, H., Fiers, W. & Grooten, J. Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses. J. Immunol. 160, 5300–5308 (1998).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
MacDonald, K. P. et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 116, 3955–3963 (2010).
Sapoznikov, A. et al. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J. Exp. Med. 204, 1923–1933 (2007).
Laouar, Y., Sutterwala, F. S., Gorelik, L. & Flavell, R. A. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 6, 600–607 (2005).
Tittel, A. P. et al. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat. Methods 9, 385–390 (2012).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Tipping, P. G. & Holdsworth, S. R. T cells in crescentic glomerulonephritis. J. Am. Soc. Nephrol. 17, 1253–1263 (2006).
Schatzmann, U., Haas, C. & Le Hir, M. A Th1 response is essential for induction of crescentic glomerulonephritis in mice. Kidney Blood Press. Res. 22, 135–139 (1999).
Shirai, T., Hirose, S., Okada, T. & Nishimura, H. in Immunological Disorders in Mice (eds Rihova, B. & Vetvicka, V.) 95–136 (CRC Press Inc., 1991).
Dubois, E. L., Horowitz, R. E., Demopoulos, H. B. & Teplitz, R. NZB/NZW mice as a model of systemic lupus erythematosus. JAMA 195, 285–289 (1966).
Rigby, R. J. et al. New loci from New Zealand black and New Zealand white mice on chromosomes 4 and 12 contribute to lupus-like disease in the context of BALB/c. J. Immunol. 172, 4609–4617 (2004).
Morel, L. et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc. Natl Acad. Sci. USA 97, 6670–6675 (2000).
Colonna, L. et al. Abnormal costimulatory phenotype and function of dendritic cells before and after the onset of severe murine lupus. Arthritis Res. Ther. 8, R49 (2006).
Rudofsky, U. H., Evans, B. D., Balaban, S. L., Mottironi, V. D. & Gabrielsen, A. E. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab. Invest. 68, 419–426 (1993).
Gu, L. et al. Genetic determinants of autoimmune disease and coronary vasculitis in the MRL-lpr/lpr mouse model of systemic lupus erythematosus. J. Immunol. 161, 6999–7006 (1998).
Anders, H. J. et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Faslpr mice. FASEB J. 18, 534–536 (2004).
Zheng, D. et al. Lipopolysaccharide-pretreated plasmacytoid dendritic cells ameliorate experimental chronic kidney disease. Kidney Int. 81, 892–902 (2012).
Huang, X. R. et al. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin. Exp. Immunol. 109, 134–142 (1997).
Triantafyllopoulou, A. et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc. Natl Acad. Sci. USA 107, 3012–3017 (2010).
Bethunaickan, R. et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J. Immunol. 186, 4994–5003 (2011).
Menke, J. et al. Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J. Am. Soc. Nephrol. 20, 2581–2592 (2009).
Teichmann, L. L. et al. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity 33, 967–978 (2010).
Acknowledgements
The work of the authors is supported by the NIH grants R01HL108954 and R01HL112914 (J.S.I.), and R01AI67541 and U01AI51698 (A.W.T.); the University of Pittsburgh O'Brien Kidney Pilot Award (J.S.I.); the Cunningham Trust, the Mrs A. E. Hogg Charitable Trust, Kidney Research UK and the UK Medical Research Council (J.H.); an American Heart Association Postdoctoral Award (13POST14520003) and an American Society of Transplantation/Pfizer Basic Science Fellowship (N.M.R.); and a Wellcome Trust Intermediate fellowship (D.A.F.). The work of J.S.I. is also supported by the Institute for Transfusion Medicine, the Haemophilia Center of Western Pennsylvania, and the Vascular Medicine Institute.
Author information
Authors and Affiliations
Contributions
J.S.I. contributed substantially to discussion of content and reviewed/edited the manuscript before submission. All other authors made substantial contributions to all stages of the preparation of the manuscript for submission. A.W.T and J.H. contributed equally as senior co-authors.
Corresponding author
Ethics declarations
Competing interests
J.S.I. is Chair of the Scientific Advisory Boards of Radiation Control Technologies and Vasculox. A.W.T. is co-inventor of a US patent (6,224,859 B1) for the generation of tolerogenic dendritic cells to promote transplant tolerance. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Rogers, N., Ferenbach, D., Isenberg, J. et al. Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat Rev Nephrol 10, 625–643 (2014). https://doi.org/10.1038/nrneph.2014.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.170