Abstract
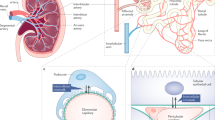
Loss of glomerular function associated with the presence of tubulointerstitial lesions, which are characterized by peritubular capillary loss, is a common finding in progressive renal disorders. Dysregulated expression of angiogenic factors (such as vascular endothelial growth factor [VEGF] and angiopoietins) and endogenous angiogenic inhibitors (such as thrombospondin-1, angiostatin and endostatin) underlie these conditions and negatively influence the balance between capillary formation and regression, resulting in capillary rarefaction. Recent studies have provided unequivocal evidence for a pathogenic role of tubulointerstitial hypoxia and the involvement of hypoxia-inducible transcription factors in the advanced stages of chronic kidney disease. The mainstay of potential angiogenic therapies is the application of angiogenic factors with the primary aim of ameliorating reduced oxygenation in the ischaemic tubulointerstitium. However, this strategy is strongly associated with inflammation and changes in vascular permeability. For example, supraphysiological expression of VEGF results in glomerular expansion and proteinuria, whereas VEGF blockade using neutralizing antibodies can cause hypertension and thrombotic microangiopathy. These effects highlight the importance of tight regulation of angiogenic factors and inhibitors. Novel therapeutic approaches that target vascular maturation and normalization are now being developed to protect kidneys from capillary rarefaction and hypoxic injury.
Key Points
-
Loss of peritubular capillaries is common during the advanced stages of chronic kidney disease (CKD) and is associated with dysregulated expression of angiogenic factors and angiogenic inhibitors
-
In the tubulointerstitium, the cellular hypoxia response mediated by hypoxia-inducible factor has both pathogenic and protective effects and chronic hypoxia leads to end-stage renal disease
-
Treatment with angiogenic factors, such as vascular endothelial growth factor (VEGF) and angiopoietin 1, prevent capillary rarefaction and ameliorate ischaemic tubulointerstitial injury in several animal models of CKD
-
VEGF-induced angiogenesis is associated with increased vascular permeability and local inflammation, which together with hypoxia, might aggravate tubulointerstitial injury
-
Data from clinical studies of anti-VEGF therapy suggest a pathogenic role of VEGF blockade in hypertension and thrombotic microangiopathy and highlight the potential risks of inappropriate control of angiogenic factors
-
Novel angiogenic therapies that target vascular maturation and normalization and might protect kidneys from tubulointerstitial injury are being developed
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Coresh, J., Astor, B. C., Greene, T., Eknoyan, G. & Levey, A. S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 41, 1–12 (2003).
Risdon, R. A., Sloper, J. C. & De Wardener, H. E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2, 363–366 (1968).
Brezis, M. & Rosen, S. Hypoxia of the renal medulla—its implications for disease. N. Engl. J. Med. 332, 647–655 (1995).
Schurek, H. J., Jost, U., Baumgärtl, H., Bertram, H. & Heckmann, U. Evidence for a preglomerular oxygen diffusion shunt in rat renal cortex. Am. J. Physiol. 259, F910–F915 (1990).
O'Connor, P. M., Anderson, W. P., Kett, M. M. & Evans, R. G. Renal preglomerular arterial-venous O2 shunting is a structural antioxidant defence mechanism of the renal cortex. Clin. Exp. Pharmacol. Physiol. 33, 637–641 (2006).
Kida, Y. & Duffield, J. S. Pivotal role of pericytes in kidney fibrosis. Clin. Exp. Pharmacol. Physiol. 38, 467–473 (2011).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Ferrara, N. VEGF-A: a critical regulator of blood vessel growth. Eur. Cytokine Netw. 20, 158–163 (2009).
Ferrara, N. & Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 18, 4–25 (1997).
Peiris-Pagès, M. The role of VEGF 165b in pathophysiology. Cell Adh. Migr. 6, 561–568 (2012).
Phng, L. K. et al. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev. Cell 16, 70–82 (2009).
Sakamoto, I. et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int. 75, 828–838 (2009).
Suzuki, Y. et al. Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int. 81, 865–879 (2012).
Lee, A. S. et al. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int. 83, 50–62 (2012).
Bry, M. et al. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation 122, 1725–1733 (2010).
Schwartz, J. D., Rowinsky, E. K., Youssoufian, H., Pytowski, B. & Wu, Y. Vascular endothelial growth factor receptor-1 in human cancer: concise review and rationale for development of IMC-18F1 (Human antibody targeting vascular endothelial growth factor receptor-1). Cancer 116, 1027–1032 (2010).
Jin, J. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151, 384–399 (2012).
Levy, A. P., Levy, N. S., Wegner, S. & Goldberg, M. A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J. Biol. Chem. 270, 13333–13340 (1995).
Levy, A. P., Levy, N. S. & Goldberg, M. A. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J. Biol. Chem. 271, 2746–2753 (1996).
Takagi, H., King, G. L., Robinson, G. S., Ferrara, N. & Aiello, L. P. Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest. Ophthalmol. Vis. Sci. 37, 2165–2176 (1996).
Augustin, H. G., Koh, G. Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Olsen, M. W. et al. Angiopoietin-4 inhibits angiogenesis and reduces interstitial fluid pressure. Neoplasia 8, 364–372 (2006).
Xu, Y., Liu, Y. J. & Yu, Q. Angiopoietin-3 inhibits pulmonary metastasis by inhibiting tumor angiogenesis. Cancer Res. 64, 6119–6126 (2004).
Lee, H. J. et al. Biological characterization of angiopoietin-3 and angiopoetin-4. FASEB J. 18, 1200–1208 (2004).
Fukuhara, S. et al. Differential function of Tie2 at cell–cell contacts and cell–substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 10, 513–526 (2008).
Saharinen, P. et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell–matrix contacts. Nat. Cell Biol. 10, 527–537 (2008).
Kidoya, H. et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 27, 522–534 (2008).
Kang, D. H. et al. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 13, 806–816 (2002).
Bohle, A., Mackensen-Haen, S. & Wehrmann, M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press. Res. 19, 191–195 (1996).
Kang, D. H., Hughes, J., Mazzali, M., Schreiner, G. F. & Johnson, R. J. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J. Am. Soc. Nephrol. 12, 1448–1457 (2001).
Chawla, L. S. & Kimmel, P. L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 82, 516–524 (2012).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
Ishii, Y. et al. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 67, 321–332 (2005).
Sutton, T. A., Fisher, C. J. & Molitoris, B. A. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 62, 1539–1549 (2002).
Molitoris, B. A. & Sutton, T. A. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 66, 496–499 (2004).
Basile, D. P., Donohoe, D., Roethe, K. & Osborn, J. L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 281, F887–F899 (2001).
Horbelt, M. et al. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am. J. Physiol. Renal Physiol. 293, F688–F695 (2007).
Kong, T., Eltzschig, H. K., Karhausen, J., Colgan, S. P. & Shelley, C. S. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of β2 integrin gene expression. Proc. Natl Acad. Sci. USA 101, 10440–10445 (2004).
Nangaku, M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 17, 17–25 (2006).
Matsumoto, M. et al. Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J. Am. Soc. Nephrol. 15, 1574–1581 (2004).
Manotham, K. et al. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J. Am. Soc. Nephrol. 15, 1277–1288 (2004).
Grgic, I. et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 82, 172–183 (2012).
Priyadarshi, A. et al. Effects of reduction of renal mass on renal oxygen tension and erythropoietin production in the rat. Kidney Int. 61, 542–546 (2002).
Palm, F., Buerk, D. G., Carlsson, P. O., Hansell, P. & Liss, P. Reduced nitric oxide concentration in the renal cortex of streptozotocin-induced diabetic rats: effects on renal oxygenation and microcirculation. Diabetes 54, 3282–3287 (2005).
Goldfarb, M. et al. Acute-on-chronic renal failure in the rat: functional compensation and hypoxia tolerance. Am. J. Nephrol. 26, 22–33 (2006).
Tanaka, T., Miyata, T., Inagi, R., Fujita, T. & Nangaku, M. Hypoxia in renal disease with proteinuria and/or glomerular hypertension. Am. J. Pathol. 165, 1979–1992 (2004).
Safran, M. et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc. Natl Acad. Sci. USA 103, 105–110 (2006).
Gloviczki, M. L. et al. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 58, 1066–1072 (2011).
Textor, S. C. et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J. Am. Soc. Nephrol. 19, 780–788 (2008).
McAllister, K. A. et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 8, 345–351 (1994).
Inoue, T. et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J. Am. Soc. Nephrol. 22, 1429–1434 (2011).
Wang, Z. J., Kumar, R., Banerjee, S. & Hsu, C. Y. Blood oxygen level-dependent (BOLD) MRI of diabetic nephropathy: preliminary experience. J. Magn. Reson. Imaging 33, 655–660 (2011).
Yin, W. J. et al. Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur. J. Radiol. 81, 1426–1431 (2012).
Brown, L. F. et al. Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 42, 1457–1461 (1992).
Simon, M. et al. Receptors of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and 125I VEGF binding sites. J. Am. Soc. Nephrol. 9, 1032–1044 (1998).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Futrakul, N., Butthep, P., Laohareungpanya, N., Chaisuriya, P. & Ratanabanangkoon, K. A defective angiogenesis in chronic kidney disease. Ren. Fail. 30, 215–217 (2008).
Di Marco, G. S. et al. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J. Am. Soc. Nephrol. 20, 2235–2245 (2009).
Masuda, Y. et al. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am. J. Pathol. 159, 599–608 (2001).
Shimizu, A. et al. Vascular endothelial growth factor165 resolves glomerular inflammation and accelerates glomerular capillary repair in rat anti-glomerular basement membrane glomerulonephritis. J. Am. Soc. Nephrol. 15, 2655–2665 (2004).
Ostendorf, T. et al. VEGF165 mediates glomerular endothelial repair. J. Clin. Invest. 104, 913–923 (1999).
Hara, A. et al. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int. 69, 1986–1995 (2006).
Leonard, E. C., Friedrich, J. L. & Basile, D. P. VEGF121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am. J. Physiol. Renal Physiol. 295, F1648–F1657 (2008).
Hohenstein, B. et al. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 69, 1654–1661 (2006).
Guo, M. et al. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J. Anat. 207, 813–821 (2005).
Liu, E. et al. Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J. Am. Soc. Nephrol. 18, 2094–2104 (2007).
Hakroush, S. et al. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am. J. Pathol. 175, 1883–1895 (2009).
Veron, D. et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 77, 989–999 (2010).
Ku, C. H. et al. Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes 57, 2824–2833 (2008).
Sivaskandarajah, G. A. et al. Vegfa protects the glomerular microvasculature in diabetes. Diabetes 61, 2958–2966 (2012).
Lindenmeyer, M. T. et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J. Am. Soc. Nephrol. 18, 1765–1776 (2007).
Woolf, A. S., Gnudi, L. & Long, D. A. Roles of angiopoietins in kidney development and disease. J. Am. Soc. Nephrol. 20, 239–244 (2009).
Yuan, H. T., Suri, C., Landon, D. N., Yancopoulos, G. D. & Woolf, A. S. Angiopoietin-2 is a site-specific factor in differentiation of mouse renal vasculature. J. Am. Soc. Nephrol. 11, 1055–1066 (2000).
Kolatsi-Joannou, M., Li, X. Z., Suda, T., Yuan, H. T. & Woolf, A. S. Expression and potential role of angiopoietins and Tie-2 in early development of the mouse metanephros. Dev. Dyn. 222, 120–126 (2001).
Satchell, S. C., Anderson, K. L. & Mathieson, P. W. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J. Am. Soc. Nephrol. 15, 566–574 (2004).
Davis, B. et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J. Am. Soc. Nephrol. 18, 2320–2329 (2007).
Futrakul, N., Butthep, P. & Futrakul, P. Altered vascular homeostasis in chronic kidney disease. Clin. Hemorheol. Microcirc. 38, 201–207 (2008).
Yuan, H. T., Tipping, P. G., Li, X. Z., Long, D. A. & Woolf, A. S. Angiopoietin correlates with glomerular capillary loss in anti-glomerular basement membrane glomerulonephritis. Kidney Int. 61, 2078–2089 (2002).
Kim, W. et al. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J. Am. Soc. Nephrol. 17, 2474–2483 (2006).
ten Dijke, P., Goumans, M. J. & Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 11, 79–89 (2008).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 (2004).
Levine, R. J. et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 355, 992–1005 (2006).
Venkatesha, S. et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649 (2006).
Rodriguez-Pena, A. et al. Endoglin upregulation during experimental renal interstitial fibrosis in mice. Hypertension 40, 713–720 (2002).
Kang, D. H. et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J. Am. Soc. Nephrol. 12, 1434–1447 (2001).
Hugo, C. & Daniel, C. Thrombospondin in renal disease. Nephron Exp. Nephrol. 111, e61–e66 (2009).
Vailhe, B. & Feige, J. J. Thrombospondins as anti-angiogenic therapeutic agents. Curr. Pharm. Des. 9, 583–588 (2003).
O'Reilly, M. S. et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 (1994).
Sima, J., Zhang, S. X., Shao, C., Fant, J. & Ma, J. X. The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett. 564, 19–23 (2004).
Zhang, S. X. et al. Therapeutic potential of angiostatin in diabetic nephropathy. J. Am. Soc. Nephrol. 17, 475–486 (2006).
Anand, S. & Cheresh, D. A. MicroRNA-mediated regulation of the angiogenic switch. Curr. Opin. Hematol. 18, 171–176 (2011).
Ghosh, G. et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J. Clin. Invest. 120, 4141–4154 (2010).
Png, K. J., Halberg, N., Yoshida, M. & Tavazoie, S. F. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 481, 190–194 (2012).
Mimura, I. et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol. Cell Biol. 32, 3018–3032 (2012).
Kaelin, W. G. Jr & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Mole, D. R. et al. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 284, 16767–16775 (2009).
Wiesener, M. S. et al. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 17, 271–273 (2003).
Warnecke, C. et al. The specific contribution of hypoxia-inducible factor-2α to hypoxic gene expression in vitro is limited and modulated by cell type-specific and exogenous factors. Exp. Cell Res. 314, 2016–2027 (2008).
Morita, M. et al. HLF/HIF-2α is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 22, 1134–1146 (2003).
Rosenberger, C. et al. Expression of hypoxia-inducible factor-1α and -2α in hypoxic and ischemic rat kidneys. J. Am. Soc. Nephrol. 13, 1721–1732 (2002).
Singh, P. et al. Aberrant tubuloglomerular feedback and HIF-1α confer resistance to ischemia after subtotal nephrectomy. J. Am. Soc. Nephrol. 23, 483–493 (2012).
Rosenberger, C. et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 73, 34–42 (2008).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Rudnicki, M. et al. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab. Invest. 89, 337–346 (2009).
Chade, A. R. et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119, 547–557 (2009).
Katavetin, P. et al. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J. Am. Soc. Nephrol. 17, 1405–1413 (2006).
Ceradini, D. J. et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J. Biol. Chem. 283, 10930–10938 (2008).
Chiang, C. K., Tanaka, T., Inagi, R., Fujita, T. & Nangaku, M. Indoxyl sulfate, a representative uremic toxin, suppresses erythropoietin production in a HIF-dependent manner. Lab. Invest. 91, 1564–1571 (2011).
Bernhardt, W. M. et al. Organ protection by hypoxia and hypoxia-inducible factors. Methods Enzymol. 435, 221–245 (2007).
Tanaka, T. & Nangaku, M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1α in progression of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 19, 43–50 (2010).
Tanaka, T. et al. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab. Invest. 85, 1292–1307 (2005).
Song, Y. R. et al. Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol. Dial. Transplant. 25, 77–85 (2010).
Yu, X. et al. Transient hypoxia-inducible factor activation in rat renal ablation and reduced fibrosis with L-mimosine. Nephrology (Carlton) 17, 58–67 (2012).
Tanaka, T. et al. Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int. 68, 2714–2725 (2005).
Kobayashi, H. et al. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J. Immunol. 188, 5106–5115 (2012).
Schley, G. et al. Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J. Am. Soc. Nephrol. 22, 2004–2015 (2011).
Kojima, I. et al. Protective role of hypoxia-inducible factor-2α against ischemic damage and oxidative stress in the kidney. J. Am. Soc. Nephrol. 18, 1218–1226 (2007).
Chade, A. R. & Kelsen, S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am. J. Physiol. Renal Physiol. 302, F1342–F1350 (2012).
Isner, J. M. et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 348, 370–374 (1996).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Claesson-Welsh, L. & Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 273, 114–127 (2013).
Flyvbjerg, A. et al. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes 51, 3090–3094 (2002).
Yamamoto, Y. et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes 53, 1831–1840 (2004).
Ichinose, K. et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 54, 2891–2903 (2005).
Grutzmacher, C. et al. Aberrant production of extracellular matrix proteins and dysfunction in kidney endothelial cells with short duration of diabetes. Am. J. Physiol. Renal Physiol. (2012).
LeCouter, J. et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412, 877–884 (2001).
Reinders, M. E. et al. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Invest. 112, 1655–1665 (2003).
Luttun, A. et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 8, 831–840 (2002).
Brigati, C., Noonan, D. M., Albini, A. & Benelli, R. Tumors and inflammatory infiltrates: friends or foes? Clin. Exp. Metastasis 19, 247–258 (2002).
Long, D. A. et al. Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int. 74, 300–309 (2008).
Aplin, A. C., Gelati, M., Fogel, E., Carnevale, E. & Nicosia, R. F. Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol. Genomics 27, 20–28 (2006).
De Palma, M. et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226 (2005).
Lemieux, C. et al. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 105, 1523–1530 (2005).
Mu, W. et al. Angiostatin overexpression is associated with an improvement in chronic kidney injury by an anti-inflammatory mechanism. Am. J. Physiol. Renal Physiol. 296, F145–F152 (2009).
Nakao, S. et al. Larger therapeutic window for steroid versus VEGF-A inhibitor in inflammatory angiogenesis: surprisingly similar impact on leukocyte infiltration. Invest. Ophthalmol. Vis. Sci. 53, 3296–3302 (2012).
Nakao, S. et al. Intravitreal anti-VEGF therapy blocks inflammatory cell infiltration and re-entry into the circulation in retinal angiogenesis. Invest. Ophthalmol. Vis. Sci. 53, 4323–4328 (2012).
Tatar, O. et al. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch. Ophthalmol. 126, 782–790 (2008).
Henry, T. D. et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am. Heart J. 142, 872–880 (2001).
Reitsma, S., Slaaf, D. W., Vink, H., van Zandvoort, M. A. & oude Egbrink, M. G. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454, 345–359 (2007).
Shibata, S., Sasaki, T., Harpel, P. & Fillit, H. Autoantibodies to vascular heparan sulfate proteoglycan in systemic lupus erythematosus react with endothelial cells and inhibit the formation of thrombin-antithrombin III complexes. Clin. Immunol. Immunopathol. 70, 114–123 (1994).
Eto, N. et al. Protection of endothelial cells by dextran sulfate in rats with thrombotic microangiopathy. J. Am. Soc. Nephrol. 16, 2997–3005 (2005).
Elson, D. A. et al. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1α. Genes Dev. 15, 2520–2532 (2001).
Mazzone, M. et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851 (2009).
Westerweel, P. E. et al. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am. J. Physiol. Renal Physiol. 292, F1132–F1140 (2007).
Chade, A. R. et al. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells 28, 1039–1047 (2010).
Goligorsky, M. S., Yasuda, K. & Ratliff, B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J. Am. Soc. Nephrol. 21, 911–919 (2010).
Bahlmann, F. H. et al. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin α persistently activates endothelial Akt and attenuates progressive organ failure. Circulation 110, 1006–1012 (2004).
Cantaluppi, V. et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 82, 412–427 (2012).
Bruno, S. et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067 (2009).
Gatti, S. et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 26, 1474–1483 (2011).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Zhuang, G. et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 31, 3513–3523 (2012).
Acknowledgements
The work of the authors is supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science 24390213 (M. Nangaku.) and 22790781 (T. Tanaka).
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, wrote the manuscript and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tanaka, T., Nangaku, M. Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol 9, 211–222 (2013). https://doi.org/10.1038/nrneph.2013.35
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.35
This article is cited by
-
In vivo imaging of renal microvasculature in a murine ischemia–reperfusion injury model using optical coherence tomography angiography
Scientific Reports (2023)
-
Identifying key genes related to the peritubular capillary rarefaction in renal interstitial fibrosis by bioinformatics
Scientific Reports (2023)
-
Reduction of renal interstitial fibrosis by targeting Tie2 in vascular endothelial cells
Pediatric Research (2023)
-
Angiogenic signaling pathways and anti-angiogenic therapy for cancer
Signal Transduction and Targeted Therapy (2023)
-
Sestrin2 remedies podocyte injury via orchestrating TSP-1/TGF-β1/Smad3 axis in diabetic kidney disease
Cell Death & Disease (2022)