Key Points
-
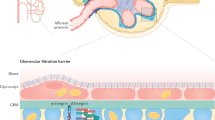
Podocytes respond to hormones and growth factors that are present in the circulation or locally produced in the glomerulus
-
A variety of receptors on the cell surface are thought to enable podocytes to respond to these external stimuli
-
The podocyte response to growth factors does not involve cell proliferation, but does include mitosis and hypertrophic cell growth, eventually leading to pathological alterations
-
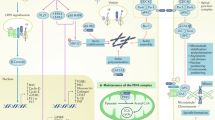
Growth factors signal via receptor tyrosine kinases (RTKs), which are promising targets for cancer therapy
-
To understand and interfere with the pathological effects of growth factors on podocytes and the glomerular filter, their precise receptors must be identified and characterized
-
RTK inhibitors that are already used in cancer therapy might be promising new treatment options for proteinuric kidney diseases
Abstract
The mammalian kidney filtration barrier is a complex multicellular, multicomponent structure that maintains homeostasis by regulating electrolytes, acid–base balance, and blood pressure (via maintenance of salt and water balance). To perform these multiple functions, podocytes—an important component of the filtration apparatus—must process a series of intercellular signals. Integrating these signals with diverse cellular responses enables a coordinated response to various conditions. Although mature podocytes are terminally differentiated and cannot proliferate, they are able to respond to growth factors. It is possible that the initial response of podocytes to growth factors is beneficial and protective, and might include the induction of hypertrophic cell growth. However, extended and/or uncontrolled growth factor signalling might be maladaptive and could result in the induction of apoptosis and podocyte loss. Growth factors signal via the activation of receptor tyrosine kinases (RTKs) on their target cells and around a quarter of the 58 RTK family members that are encoded in the human genome have been identified in podocytes. Pharmacological inhibitors of many RTKs exist and are currently used in experimental and clinical cancer therapy. The identification of pathological RTK-mediated signal transduction pathways in podocytes could provide a starting point for the development of novel therapies for glomerular disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mundel, P. & Kriz, W. Structure and function of podocytes: an update. Anat. Embryol. (Berl.) 192, 385–397 (1995).
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Neal, C. R. et al. Glomerular filtration into the subpodocyte space is highly restricted under physiological perfusion conditions. Am. J. Physiol. Renal Physiol. 293, F1787–F1798 (2007).
Greka, A. & Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 74, 299–323 (2012).
Khurana, S., Bruggeman, L. A. & Kao, H. Y. Nuclear hormone receptors in podocytes. Cell Biosci. 2, 33 (2012).
Sachs, N. & Sonnenberg, A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol. 9, 200–210 (2013).
Gomperts, B. D., Tatham, P. E. R. & Kramer, I. M. Signal Transduction 2nd edn (Elsevier Academic Press, 2009).
Langley, J. N. On the physiology of the salivary secretion: part II. On the mutual antagonism of atropin and pilocarpin, having especial reference to their relations in the sub-maxillary gland of the cat. J. Physiol. 1, 339–369 (1878).
Davenport, H. W. Early history of the concept of chemical transmission of the nerve impulse. Physiologist 34, 178–190 (1991).
Cohen, P. The regulation of protein function by multisite phosphorylation—a 25 year update. Trends Biochem. Sci. 25, 596–601 (2000).
Collett, M. S. & Erikson, R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc. Natl Acad. Sci. USA 75, 2021–2024 (1978).
Hunter, T. & Sefton, B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl Acad. Sci. USA 77, 1311–1315 (1980).
Ushiro, H. & Cohen, S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J. Biol. Chem. 255, 8363–8365 (1980).
Kasuga, M., Karlsson, F. A. & Kahn, C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science 215, 185–187 (1982).
Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 103, 211–225 (2000).
Sadowski, I., Stone, J. C. & Pawson, T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol. Cell Biol. 6, 4396–4408 (1986).
Anderson, D. et al. Binding of SH2 domains of phospholipase C γ1, GAP, and Src to activated growth factor receptors. Science 250, 979–982 (1990).
Pawson, T. & Schlessingert, J. SH2 and SH3 domains. Curr. Biol. 3, 434–442 (1993).
Liu, B. A. et al. The human and mouse complement of SH2 domain proteins—establishing the boundaries of phosphotyrosine signaling. Mol. Cell 22, 851–868 (2006).
Karkkainen, S. et al. Identification of preferred protein interactions by phage-display of the human Src homology-3 proteome. EMBO Rep. 7, 186–191 (2006).
Pawson, T. & Nash, P. Assembly of cell regulatory systems through protein interaction domains. Science 300, 445–452 (2003).
Grassot, J., Mouchiroud, G. & Perriere, G. RTKdb: database of receptor tyrosine kinase. Nucleic Acids Res. 31, 353–358 (2003).
Lemmon, M. A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Ward, C. W. et al. The insulin and EGF receptor structures: new insights into ligand-induced receptor activation. Trends Biochem. Sci. 32, 129–137 (2007).
Witsch, E., Sela, M. & Yarden, Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 25, 85–101 (2010).
Haglund, K., Rusten, T. E. & Stenmark, H. Aberrant receptor signaling and trafficking as mechanisms in oncogenesis. Crit. Rev. Oncog. 13, 39–74 (2007).
Giamas, G. et al. Kinases as targets in the treatment of solid tumors. Cell Signal. 22, 984–1002 (2010).
Kriz, W. et al. The role of podocytes in the development of glomerular sclerosis. Kidney Int. Suppl. 45, S64–S72 (1994).
Kriz, W., Shirato, I., Nagata, M., LeHir, M. & Lemley, K. V. The podocyte's response to stress: the enigma of foot process effacement. Am. J. Physiol. Renal Physiol. 304, F333–F347 (2013).
Liapis, H., Romagnani, P. & Anders, H. J. New insights into the pathology of podocyte loss: mitotic catastrophe. Am. J. Pathol. 183, 1364–1374 (2013).
Coward, R. J. & Saleem, M. A. Podocytes as a target of insulin. Curr. Diabetes Rev. 7, 22–27 (2011).
Jauregui, A., Mintz, D. H., Mundel, P. & Fornoni, A. Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr. Opin. Nephrol. Hypertens. 18, 539–545 (2009).
Stieger, N., Worthmann, K. & Schiffer, M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes Metab. Res. Rev. 27, 207–215 (2011).
Hale, L. J. & Coward, R. J. The insulin receptor and the kidney. Curr. Opin. Nephrol. Hypertens. 22, 100–106 (2013).
Coward, R. J. et al. The human glomerular podocyte is a novel target for insulin action. Diabetes 54, 3095–3102 (2005).
Anfossi, G., Russo, I., Doronzo, G. & Trovati, M. Contribution of insulin resistance to vascular dysfunction. Arch. Physiol. Biochem. 115, 199–217 (2009).
Ritchie, S. A., Ewart, M. A., Perry, C. G., Connell, J. M. & Salt, I. P. The role of insulin and the adipocytokines in regulation of vascular endothelial function. Clin. Sci. (Lond.) 107, 519–532 (2004).
Wolf, G., Chen, S. & Ziyadeh, F. N. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54, 1626–1634 (2005).
Coward, R. J. et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56, 1127–1135 (2007).
Welsh, G. I. et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell. Metab. 12, 329–340 (2010).
Mogensen, C. E., Christensen, N. J. & Gundersen, H. J. The acute effect of insulin on heart rate, blood pressure, plasma noradrenaline and urinary albumin excretion. The role of changes in blood glucose. Diabetologia 18, 453–457 (1980).
Fogo, A. B. & Kon, V. The glomerulus—a view from the inside—the endothelial cell. Int. J. Biochem. Cell Biol. 42, 1388–1397 (2010).
Advani, A. Vascular endothelial growth factor and the kidney: something of the marvellous. Curr. Opin. Nephrol. Hypertens. 23, 87–92 (2014).
Ku, C. H. et al. Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes 57, 2824–2833 (2008).
Guan, F., Villegas, G., Teichman, J., Mundel, P. & Tufro, A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am. J. Physiol. Renal Physiol. 291, F422–F428 (2006).
Chen, S. et al. Podocyte-derived vascular endothelial growth factor mediates the stimulation of α3(IV) collagen production by transforming growth factor-β1 in mouse podocytes. Diabetes 53, 2939–2949 (2004).
Foster, R. R. et al. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am. J. Physiol. Renal Physiol. 284, F1263–F1273 (2003).
Veron, D. et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 77, 989–999 (2010).
Veron, D. et al. Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid-resistant nephrotic syndrome. Am. J. Pathol. 177, 2225–2233 (2010).
Sison, K. et al. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J. Am. Soc. Nephrol. 21, 1691–1701 (2010).
Itoh, N. & Ornitz, D. M. Evolution of the FGF and FGFR gene families. Trends Genet. 20, 563–569 (2004).
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Szebenyi, G. & Fallon, J. F. Fibroblast growth factors as multifunctional signaling factors. Int. Rev. Cytol. 185, 45–106 (1999).
Eswarakumar, V. P., Lax, I. & Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 16, 139–149 (2005).
Bates, C. M. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr. Nephrol. 22, 343–349 (2007).
Celli, G., LaRochelle, W. J., Mackem, S., Sharp, R. & Merlino, G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J. 17, 1642–1655 (1998).
Davidson, G., Dono, R. & Zeller, R. FGF signalling is required for differentiation-induced cytoskeletal reorganisation and formation of actin-based processes by podocytes. J. Cell Sci. 114, 3359–3366 (2001).
Floege, J. et al. Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J. Clin. Invest. 96, 2809–2819 (1995).
Cauchi, J. et al. Light-microscopic immunolocalization of fibroblast growth factor-1 and -2 in adult rat kidney. Cell Tissue Res. 285, 179–187 (1996).
Takeuchi, A. et al. Basic fibroblast growth factor promotes proliferation of rat glomerular visceral epithelial cells in vitro. Am. J. Pathol. 141, 107–116 (1992).
Floege, J. et al. Localization of fibroblast growth factor-2 (basic FGF) and FGF receptor-1 in adult human kidney. Kidney Int. 56, 883–897 (1999).
Floege, J. et al. Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J. Clin. Invest. 90, 2362–2369 (1992).
Floege, J. et al. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J. Clin. Invest. 92, 2952–2962 (1993).
Ballermann, B. J. Regulation of bovine glomerular endothelial cell growth in vitro. Am. J. Physiol. 256, C182–C189 (1989).
Dono, R. & Zeller, R. Cell-type-specific nuclear translocation of fibroblast growth factor-2 isoforms during chicken kidney and limb morphogenesis. Dev. Biol. 163, 316–330 (1994).
Tossidou, I. et al. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J. Biol. Chem. 285, 25285–25295 (2010).
Tossidou, I. et al. CD2AP/CIN85 balance determines receptor tyrosine kinase signaling response in podocytes. J. Biol. Chem. 282, 7457–7464 (2007).
Ray, P. E. et al. bFGF and its low affinity receptors in the pathogenesis of HIV-associated nephropathy in transgenic mice. Kidney Int. 46, 759–772 (1994).
Strutz, F. et al. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 57, 1521–1538 (2000).
Mazue, G., Bertelero, F., Garofano, L., Brughera, M. & Carminati, P. Experience with the preclinical assessment of basic fibroblast growth factor (bFGF). Toxicol. Lett. 64–65, 329–338 (1992).
Kriz, W., Hähnel, B., Rösener, S. & Elger, M. Long-term treatment of rats with FGF-2 results in focal segmental glomerulosclerosis. Kidney Int. 48, 1435–1450 (1995).
Crabtree, G. R. & Olson, E. N. NFAT signaling: choreographing the social lives of cells. Cell 109 (Suppl.), S67–S79 (2002).
Wang, Y. et al. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J. Am. Soc. Nephrol. 21, 1657–1666 (2010).
Nijenhuis, T. et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am. J. Pathol. 179, 1719–1732 (2011).
Shankland, S. J. et al. Cyclin kinase inhibitors are increased during experimental membranous nephropathy: potential role in limiting glomerular epithelial cell proliferation in vivo. Kidney Int. 52, 404–413 (1997).
Riley, S. G., Steadman, R., Williams, J. D., Floege, J. & Phillips, A. O. Augmentation of kidney injury by basic fibroblast growth factor or platelet-derived growth factor does not induce progressive diabetic nephropathy in the Goto Kakizaki model of non-insulin-dependent diabetes. J. Lab. Clin. Med. 134, 304–312 (1999).
Sasaki, T., Hatta, H. & Osawa, G. Cytokines and podocyte injury: the mechanism of fibroblast growth factor 2-induced podocyte injury. Nephrol. Dial. Transplant. 14 (Suppl. 1), 33–34 (1999).
Sasaki, T., Jyo, Y., Tanda, N., Tamai, H. & Osawa, G. The role of basic fibroblast growth factor (FGF2) in glomerular epithelial cell injury. Contrib. Nephrol. 118, 68–77 (1996).
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 (2009).
Unger, E. F. et al. Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. Am. J. Cardiol. 85, 1414–1419 (2000).
Zimering, M. B. & Eng, J. Increased basic fibroblast growth factor-like substance in plasma from a subset of middle-aged or elderly male diabetic patients with microalbuminuria or proteinuria. J. Clin. Endocrinol. Metab. 81, 4446–4452 (1996).
Ray, P. E., Liu, X. H., Xu, L. & Rakusan, T. Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr. Nephrol. 13, 586–593 (1999).
Nugent, M. A. & Iozzo, R. V. Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 32, 115–120 (2000).
Floege, J. et al. Endogenous fibroblast growth factor-2 mediates cytotoxicity in experimental mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 9, 792–801 (1998).
Nickel, W. Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 21, 621–626 (2010).
Ornitz, D. M. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22, 108–112 (2000).
Schumacher, V. A. et al. WT1-dependent sulfatase expression maintains the normal glomerular filtration barrier. J. Am. Soc. Nephrol. 22, 1286–1296 (2011).
Pelletier, J. et al. Germline mutations in the Wilm's tumor suppressor gene are associated with abnormal urogential development in Denys-Drash syndrome. Cell 67, 437–447 (1991).
Okamoto, K. et al. Common variation in GPC5 is associated with acquired nephrotic syndrome. Nat. Genet. 43, 459–463 (2011).
Bernfield, M. et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999).
Tallquist, M. & Kazlauskas, A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 15, 205–213 (2004).
Floege, J., Eitner, F. & Alpers, C. E. A new look at platelet-derived growth factor in renal disease. J. Am. Soc. Nephrol. 19, 12–23 (2008).
Floege, J. et al. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 41, 297–309 (1992).
Matsuda, M. et al. Gene expression of PDGF and PDGF receptor in various forms of glomerulonephritis. Am. J. Nephrol. 17, 25–31 (1997).
Uehara, G., Suzuki, D., Toyoda, M., Umezono, T. & Sakai, H. Glomerular expression of platelet-derived growth factor (PDGF)-A, -B chain and PDGF receptor-α, -β in human diabetic nephropathy. Clin. Exp. Nephrol. 8, 36–42 (2004).
Iida, H. et al. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc. Natl Acad. Sci. USA 88, 6560–6564 (1991).
van Roeyen, C. R. et al. Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int. 69, 1393–1402 (2006).
Ostendorf, T. et al. A fully human monoclonal antibody (CR002) identifies PDGF-D as a novel mediator of mesangioproliferative glomerulonephritis. J. Am. Soc. Nephrol. 14, 2237–2247 (2003).
Hudkins, K. L. et al. Exogenous PDGF-D is a potent mesangial cell mitogen and causes a severe mesangial proliferative glomerulopathy. J. Am. Soc. Nephrol. 15, 286–298 (2004).
Changsirikulchai, S. et al. Platelet-derived growth factor-D expression in developing and mature human kidneys. Kidney Int. 62, 2043–2054 (2002).
Gesualdo, L. et al. Expression of platelet-derived growth factor receptors in normal and diseased human kidney. An immunohistochemistry and in situ hybridization study. J. Clin. Invest. 94, 50–58 (1994).
Alpers, C. E., Seifert, R. A., Hudkins, K. L., Johnson, R. J. & Bowen-Pope, D. F. PDGF-receptor localizes to mesangial, parietal epithelial, and interstitial cells in human and primate kidneys. Kidney Int. 43, 286–294 (1993).
Bergsten, E. et al. PDGF-D is a specific, protease-activated ligand for the PDGF β-receptor. Nat. Cell Biol. 3, 512–516 (2001).
van Roeyen, C. R. et al. Induction of progressive glomerulonephritis by podocyte-specific overexpression of platelet-derived growth factor-D. Kidney Int. 80, 1292–1305 (2011).
Nakamura, T., Nawa, K. & Ichihara, A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem. Biophys. Res. Commun. 122, 1450–1459 (1984).
Nakamura, T. et al. Molecular cloning and expression of human hepatocyte growth factor. Nature 342, 440–443 (1989).
Nakamura, T. & Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 86, 588–610 (2010).
Park, M. et al. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl Acad. Sci. USA 84, 6379–6383 (1987).
Sonnenberg, E., Meyer, D., Weidner, K. M. & Birchmeier, C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J. Cell Biol. 123, 223–235 (1993).
Zhang, X. et al. Sp1 and Sp3 transcription factors synergistically regulate HGF receptor gene expression in kidney. Am. J. Physiol. Renal Physiol. 284, F82–F94 (2003).
Mizuno, S., Matsumoto, K. & Nakamura, T. HGF as a renotrophic and anti-fibrotic regulator in chronic renal disease. Front. Biosci. 13, 7072–7086 (2008).
Mizuno, S. et al. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J. Clin. Invest. 101, 1827–1834 (1998).
Dai, C. et al. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J. Am. Soc. Nephrol. 15, 2637–2647 (2004).
Cruzado, J. M. et al. Regression of advanced diabetic nephropathy by hepatocyte growth factor gene therapy in rats. Diabetes 53, 1119–1127 (2004).
Bu, X. et al. Systemic administration of naked plasmid encoding HGF attenuates puromycin aminonucleoside-induced damage of murine glomerular podocytes. Am. J. Physiol. Renal Physiol. 301, F784–F792 (2011).
Kato, T., Mizuno, S. & Nakamura, T. Preservations of nephrin and synaptopodin by recombinant hepatocyte growth factor in podocytes for the attenuations of foot process injury and albuminuria in nephritic mice. Nephrology (Carlton) 16, 310–318 (2011).
Dai, C., Saleem, M. A., Holzman, L. B., Mathieson, P. & Liu, Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 77, 962–973 (2010).
Fornoni, A., Li, H., Foschi, A., Striker, G. E. & Striker, L. J. Hepatocyte growth factor, but not insulin-like growth factor I protects podocytes against cyclosporin A-induced apoptosis. Am. J. Pathol. 158, 275–280 (2001).
Yang, J. & Liu, Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J. Am. Soc. Nephrol. 13, 96–107 (2002).
Zhang, J., Yang, J. & Liu, Y. Role of Bcl-xL induction in HGF-mediated renal epithelial cell survival after oxidant stress. Int. J. Clin. Exp. Pathol. 1, 242–253 (2008).
Yamaguchi, Y. et al. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am. J. Kidney Dis. 54, 653–664 (2009).
Li, Y. et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. Pathol. 172, 299–308 (2008).
Avraham, R. & Yarden, Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117 (2011).
Zhang, H. et al. ErbB receptors: from oncogenes to targeted cancer therapies. J. Clin. Invest. 117, 2051–2058 (2007).
Takeuchi, K. & Ito, F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol. Pharm. Bull. 34, 1774–1780 (2011).
Force, T. & Kolaja, K. L. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discov. 10, 111–126 (2011).
Nowak, G. & Schnellmann, R. G. Integrative effects of EGF on metabolism and proliferation in renal proximal tubular cells. Am. J. Physiol. 269, C1317–C1325 (1995).
Pugh, J. L., Sweeney, W. E. Jr. & Avner, E. D. Tyrosine kinase activity of the EGF receptor in murine metanephric organ culture. Kidney Int. 47, 774–781 (1995).
Zeng, F., Singh, A. B. & Harris, R. C. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 315, 602–610 (2009).
Lautrette, A. et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat. Med. 11, 867–874 (2005).
Pillebout, E. et al. JunD protects against chronic kidney disease by regulating paracrine mitogens. J. Clin. Invest. 112, 843–852 (2003).
Coaxum, S. D., Garnovskaya, M. N., Gooz, M., Baldys, A. & Raymond, J. R. Epidermal growth factor activates Na+/H+ exchanger in podocytes through a mechanism that involves Janus kinase and calmodulin. Biochim. Biophys. Acta 1793, 1174–1181 (2009).
Harris, R. C., Hoover, R. L., Jacobson, H. R. & Badr, K. F. Evidence for glomerular actions of epidermal growth factor in the rat. J. Clin. Invest. 82, 1028–1039 (1988).
Adler, S. & Eng, B. Reversal of inhibition of rat glomerular epithelial cell growth by growth factors. Am. J. Pathol. 136, 557–563 (1990).
Tassin, M. T. et al. Effects of epidermal growth factor on calf renal glomerular cells in vitro. Growth Factors 6, 243–254 (1992).
Flannery, P. J. & Spurney, R. F. Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes. Nephron Exp. Nephrol. 103, e109–e118 (2006).
Suzuki, H., Yamamoto, T., Fujigaki, Y., Eguchi, S. & Hishida, A. Comparison of ROCK and EGFR activation pathways in the progression of glomerular injuries in AngII-infused rats. Ren. Fail. 33, 1005–1012 (2011).
Chen, J., Chen, J. K., Neilson, E. G. & Harris, R. C. Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. J. Am. Soc. Nephrol. 17, 1615–1623 (2006).
Advani, A. et al. Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology (Carlton) 16, 573–581 (2011).
Feng, L. et al. Heparin-binding EGF-like growth factor contributes to reduced glomerular filtration rate during glomerulonephritis in rats. J. Clin. Invest. 105, 341–350 (2000).
Bollee, G. M. et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat. Med. 17, 1242–1250 (2011).
Sakai, M. et al. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J. Clin. Invest. 99, 2128–2138 (1997).
Oda, K., Matsuoka, Y., Funahashi, A. & Kitano, H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 1, 2005.0010 (2005).
Satchell, S. C. et al. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J. Am. Soc. Nephrol. 13, 544–550 (2002).
Davis, B. et al. Podocyte-specific induced overexpression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J. Am. Soc. Nephrol. 18, 2320–2329 (2007).
Gao, X. et al. Angiopoietin-like protein 3 regulates the motility and permeability of podocytes by altering nephrin expression in vitro. Biochem. Biophys. Res. Commun. 399, 31–36 (2010).
Jia, R., Hong, X., Li, S., Haichun, Y. & Chuanming, H. Expression of angiopoietin-like 3 associated with puromycin-induced podocyte damage. Nephron Exp. Nephrol. 115, e38–e45 (2010).
Clement, L. C. et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 17, 117–122 (2011).
Guha, M., Xu, Z. G., Tung, D., Lanting, L. & Natarajan, R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 21, 3355–3368 (2007).
Yokoi, H. et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 73, 446–455 (2008).
Fuchshofer, R. et al. Connective tissue growth factor modulates podocyte actin cytoskeleton and extracellular matrix synthesis and is induced in podocytes upon injury. Histochem. Cell Biol. 136, 301–319 (2011).
Dai, H. Y. et al. The roles of connective tissue growth factor and integrin-linked kinase in high glucose-induced phenotypic alterations of podocytes. J. Cell Biochem. 113, 293–301 (2012).
Gross, O. et al. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 66, 102–111 (2004).
Kerroch, M. et al. Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. FASEB J. 26, 4079–4091 (2012).
Hashimoto, T. et al. Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int. 72, 954–964 (2007).
Wnuk, M. et al. Podocyte EphB4 signaling helps recovery from glomerular injury. Kidney Int. 81, 1212–1225 (2012).
Hale, L. J. et al. Insulin-like growth factor-II is produced by, signals to and is an important survival factor for the mature podocyte in man and mouse. J. Pathol. 230, 95–106 (2013).
Fujinaka, H. et al. Expression and localization of insulin-like growth factor binding proteins in normal and proteinuric kidney glomeruli. Nephrology (Carlton) 15, 700–709 (2010).
Bridgewater, D. J., Ho, J., Sauro, V. & Matsell, D. G. Insulin-like growth factors inhibit podocyte apoptosis through the PI3 kinase pathway. Kidney Int. 67, 1308–1314 (2005).
Bridgewater, D. J., Dionne, J. M., Butt, M. J., Pin, C. L. & Matsell, D. G. The role of the type I insulin-like growth factor receptor (IGF-IR) in glomerular integrity. Growth Horm. IGF Res. 18, 26–37 (2008).
Prabakaran, T. et al. Receptor-mediated endocytosis of α-galactosidase A in human podocytes in Fabry disease. PLoS ONE 6, e25065 (2011).
Hale, L. J. et al. Insulin directly stimulates VEGF-A production in the glomerular podocyte. Am. J. Physiol. Renal Physiol. 305, F182–F188 (2013).
Kim, E. Y., Anderson, M. & Dryer, S. E. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am. J. Physiol. Renal Physiol. 302, F298–F307 (2012).
Carito, V. et al. Localization of nerve growth factor (NGF) receptors in the mitochondrial compartment: characterization and putative role. Biochim. Biophys. Acta 1820, 96–103 (2012).
Hahn, W. H., Suh, J. S. & Cho, B. S. Linkage and association study of neurotrophins and their receptors as novel susceptibility genes for childhood IgA nephropathy. Pediatr. Res. 69, 299–305 (2011).
Tsui, C. C., Shankland, S. J. & Pierchala, B. A. Glial cell line-derived neurotrophic factor and its receptor ret is a novel ligand-receptor complex critical for survival response during podocyte injury. J. Am. Soc. Nephrol. 17, 1543–1552 (2006).
Benz, K. et al. Early glomerular alterations in genetically determined low nephron number. Am. J. Physiol. Renal Physiol. 300, F521–F530 (2011).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 (2003).
Acknowledgements
J. Reiser's research is supported by NIH grants DK073495, DK089394, DK093773 and DK101350. S. Sever's research is funded by NIH grant DK087985. C. Faul's research is funded by a Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology.
Author information
Authors and Affiliations
Contributions
J. Reiser and C. Faul researched the data and wrote the article. All authors made a substantial contribution to discussions of the content and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
J. Reiser has issued and pending patents on the development of novel kidney protective therapeutics. He stands to gain royalties from their commercialization. S. Sever and C. Faul declare no competing interests.
Rights and permissions
About this article
Cite this article
Reiser, J., Sever, S. & Faul, C. Signal transduction in podocytes—spotlight on receptor tyrosine kinases. Nat Rev Nephrol 10, 104–115 (2014). https://doi.org/10.1038/nrneph.2013.274
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.274
This article is cited by
-
Insulin-activated store-operated Ca2+ entry via Orai1 induces podocyte actin remodeling and causes proteinuria
Nature Communications (2021)
-
Role of actin cytoskeleton in podocytes
Pediatric Nephrology (2021)
-
Optimal use of lenvatinib in the treatment of advanced thyroid cancer
Cancers of the Head & Neck (2017)
-
Down-regulation of PAX2 promotes in vitro differentiation of podocytes from human CD34+ cells
Cell and Tissue Research (2017)
-
Lipotoxicity as a trigger factor of renal disease
Journal of Nephrology (2016)