Key Points
-
Incretin-based therapies—glucagon-like peptide 1 receptor (GLP-1R) agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors—improve glycaemic control by ameliorating multiple phenotypic defects associated with type 2 diabetes mellitus
-
GLP-1R agonists reduce body weight, whereas DPP-4 inhibitors do not affect body weight; both are generally well-tolerated by patients
-
Evidence from animal and human studies indicates that incretin-based therapies might prevent the onset and progression of diabetic nephropathy, as measured by clinical and histological improvements
-
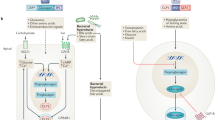
Incretin-based therapies might positively influence haemodynamic variables (hyperfiltration, glomerular capillary hydraulic pressure, and systemic blood pressure), metabolic factors (glycaemia, dyslipidaemia, oxidative stress) and inflammatory pathways in the pathogenesis of diabetic nephropathy
-
Inhibitors of DPP-4 block the degradation of endogenous GLP-1 and might also influence circulating levels and activity of other vasoactive peptides that could act on the kidney
Abstract
Diabetic nephropathy is the leading cause of end-stage renal disease worldwide, and is associated with a high risk of cardiovascular morbidity and mortality. Intensive control of glucose levels and blood pressure is currently the mainstay of both prevention and treatment of diabetic nephropathy. However, this strategy cannot fully prevent the development and progression of diabetic nephropathy, and an unmet need remains for additional novel therapies. The incretin-based agents—agonists of glucagon-like peptide 1 receptor (GLP-1R) and inhibitors of dipeptidyl peptidase 4 (DPP-4), an enzyme that degrades glucagon-like peptide 1—are novel blood-glucose-lowering drugs used in the treatment of type 2 diabetes mellitus (T2DM). Therapeutic agents from these two drug classes improve pancreatic islet function and induce extrapancreatic effects that ameliorate various phenotypic defects of T2DM that are beyond glucose control. Agonists of GLP-1R and inhibitors of DPP-4 reduce blood pressure, dyslipidaemia and inflammation, although only GLP-1R agonists decrease body weight. Both types of incretin-based agents inhibit renal tubular sodium reabsorption and decrease glomerular pressure as well as albuminuria in rodents and humans. In rodents, incretin-based therapies also prevent onset of the morphological abnormalities of diabetic nephropathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
02 May 2014
It is with regret that Nature Review Nephrology informs readers that Professor Michaela Diamant passed away on 9 April 2014. Future correspondence for this article should be addressed to Marcel H. A. Muskiet (ma.muskiet@vumc.nl).
References
Whiting, D. R., Guariguata, L., Weil, C. & Shaw, J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 94, 311–321 (2011).
Williams, M. E. Diabetic CKD/ESRD 2010: a progress report? Semin. Dial. 23, 129–133 (2010).
Ninomiya, T. et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 20, 1813–1821 (2009).
Afkarian, M. et al. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 24, 302–308 (2013).
Jager, A. et al. Microalbuminuria is strongly associated with NIDDM and hypertension, but not with the insulin resistance syndrome: the Hoorn Study. Diabetologia 41, 694–700 (1998).
Turner, R. C. et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316, 823–828 (1998).
Ismail, N., Becker, B., Strzelczyk, P. & Ritz, E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 55, 1–28 (1999).
Hamet, P. What matters in ADVANCE and ADVANCE-ON. Diabetes Obes. Metab. 14 (Suppl. 1), 20–29 (2012).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393 (2003).
Ruggenenti, P. & Remuzzi, G. Nephropathy of type 1 and type 2 diabetes: diverse pathophysiology, same treatment? Nephrol. Dial. Transplant. 15, 1900–1902 (2000).
Cooper, M. E. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 44, 1957–1972 (2001).
Barnes, D., Pinto, J. & Viberti, G. in Oxford Textbook of Clinical Nephrology (ed. Davison, A.) 723–775 (Oxford University Press, 1998).
Helal, I., Fick-Brosnahan, G. M., Reed-Gitomer, B. & Schrier, R. W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8, 293–300 (2012).
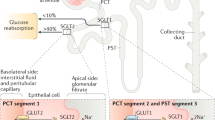
Vallon, V. & Thomson, S. C. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu. Rev. Physiol. 74, 351–375 (2012).
Fioretto, P. & Mauer, M. Histopathology of diabetic nephropathy. Semin. Nephrol. 27, 195–207 (2007).
Frische, S. Glomerular filtration rate in early diabetes: ongoing discussions of causes and mechanisms. J. Nephrol. 24, 537–540 (2011).
Saad, S. et al. High glucose transactivates the EGF receptor and up-regulates serum glucocorticoid kinase in the proximal tubule. Kidney Int. 68, 985–997 (2005).
Ruggenenti, P., Cravedi, P. & Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 6, 319–330 (2010).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107, 1058–1070 (2010).
Du, X. L. et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl Acad. Sci. USA 97, 12222–12226 (2000).
Nishikawa, T. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790 (2000).
Navarro-González, J. F., Mora-Fernández, C., Muros de Fuentes, M. & García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 7, 327–340 (2011).
Poulsen, P. L., Hansen, K. W. & Mogensen, C. E. Ambulatory blood pressure in the transition from normo- to microalbuminuria. A longitudinal study in IDDM patients. Diabetes 43, 1248–1253 (1994).
Rosario, R. F. & Prabhakar, S. Lipids and diabetic nephropathy. Curr. Diab. Rep. 6, 455–462 (2006).
Ziyadeh, F. N. & Wolf, G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr. Diabetes Rev. 4, 39–45 (2008).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Chapter 1: definition and classification of CKD. Kidney Int. Suppl. 3, 19–62 (2013).
Abbate, M., Zoja, C. & Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 17, 2974–2984 (2006).
Adler, A. I. et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 63, 225–232 (2003).
National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am. J. Kidney Dis. 60, 850–886 (2012).
Kramer, H. J., Nguyen, Q. D., Curhan, G. & Hsu, C.-Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289, 3273–3277 (2003).
Basi, S., Fesler, P., Mimran, A. & Lewis, J. B. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabetes Care 31 (Suppl. 2), S194–S201 (2008).
Perkins, B. A., Ficociello, L. H., Roshan, B., Warram, J. H. & Krolewski, A. S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 77, 57–64 (2010).
Heerspink, H. J. & de Zeeuw, D. The kidney in type 2 diabetes therapy. Rev. Diabet. Stud. 8, 392–402 (2011).
Wang, P. H., Lau, J. & Chalmers, T. C. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet 341, 1306–1309 (1993).
[No authors listed] The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 329, 977–986 (1993).
De Boer, I. H. et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N. Engl. J. Med. 365, 2366–2376 (2011).
Nathan, D. M. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353, 2643–2653 (2005).
[No authors listed] Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 837–853 (1998).
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
Skyler, J. S. et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology. Circulation 119, 351–357 (2009).
Gerstein, H. C. et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008).
Ismail-Beigi, F. et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376, 419–430 (2010).
Patel, A. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
Agrawal, L. et al. Observation on renal outcomes in the Veterans Affairs Diabetes Trial. Diabetes Care 34, 2090–2094 (2011).
Coca, S. G., Ismail-Beigi, F., Haq, N., Krumholz, H. M. & Parikh, C. R. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch. Intern. Med. 172, 761–769 (2012).
Perkovic, V. et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 83, 517–523 (2013).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–1379 (2012).
[No authors listed] Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317, 703–713 (1998).
Holman, R. R., Paul, S. K., Bethel, M. A., Neil, H. A. & Matthews, D. R. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N. Engl. J. Med. 359, 1565–1576 (2008).
Patel, A. et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370, 829–840 (2007).
Cushman, W. C. et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1575–1585 (2010).
Ismail-Beigi, F. et al. Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int. 81, 586–594 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. Suppl. 2, 337–414 (2012).
Gaede, P., Lund-Andersen, H., Parving, H.-H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591 (2008).
Lewis, E. J. et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
[No authors listed] Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 349, 1787–1792 (1997).
Mauer, M. et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 361, 40–51 (2009).
Bilous, R. et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann. Intern. Med. 151, 11–20 (2009).
[No authors listed] Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 355, 253–259 (2000).
Ruggenenti, P. et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 351, 1941–1951 (2004).
Haller, H. et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med. 364, 907–917 (2011).
Parving, H. H. et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N. Engl. J. Med. 345, 870–878 (2001).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Yusuf, S. et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 372, 1174–1183 (2008).
Mallat, S. G. Dual renin-angiotensin system inhibition for prevention of renal and cardiovascular events: do the latest trials challenge existing evidence? Cardiovasc. Diabetol. 12, 108 (2013).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Parving, H.-H. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213 (2012).
Fried, L. F. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369, 1892–1903 (2013).
Bakris, G. L. et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J. Hypertens. 24, 2047–2055 (2006).
Schernthaner, G., Matthews, D. R., Charbonnel, B., Hanefeld, M. & Brunetti, P. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J. Clin. Endocrinol. Metab. 89, 6068–6076 (2004).
European Medicines Agency Press Office. European Medicines Agency recommends suspension of avandia, avandamet and avaglim. Anti-diabetes medication to be taken off the market. [online] (2010).
Schernthaner, G., Currie, C. J. & Schernthaner, G.-H. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care 36 (Suppl. 2), S155–S161 (2013).
Eissele, R. et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Invest. 22, 283–291 (1992).
Kreymann, B., Williams, G., Ghatei, M. A. & Bloom, S. R. Glucagon-like peptide-17–36: a physiological incretin in man. Lancet 2, 1300–1304 (1987).
Fineman, M. S., Cirincione, B. B., Maggs, D. & Diamant, M. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes. Obes. Metab. 14, 675–688 (2012).
Elrick, H., Stimmler, L., Hlad, C. J. & Arai, Y. Plasma insulin response to oral and intravenous glucose administration. J. Clin. Endocrinol. Metab. 24, 1076–1082 (1964).
McIntyre, N., Holdsworrth, C. D. & Turner, D. S. New interpretation of oral glucose tolerance. Lancet 2, 20–21 (1964).
Nauck, M. A. et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 63, 492–498 (1986).
Nauck, M., Stöckmann, F., Ebert, R. & Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29, 46–52 (1986).
Nauck, M. A. et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 17–36 amide in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36, 741–744 (1993).
Zander, M., Madsbad, S., Madsen, J. L. & Holst, J. J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 359, 824–830 (2002).
Mentlein, R., Gallwitz, B. & Schmidt, W. E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-17–36 amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 214, 829–835 (1993).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Eng, J., Kleinman, W. A., Singh, L., Singh, G. & Raufman, J. P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 267, 7402–7405 (1992).
Aroda, V. R. et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin. Ther. 34, 1247–1258 (2012).
Mayo, K. E. et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 55, 167–194 (2003).
Thorens, B. Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl Acad. Sci. USA 89, 8641–8645 (1992).
Sivertsen, J., Rosenmeier, J., Holst, J. J. & Vilsbøll, T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat. Rev. Cardiol. 9, 209–222 (2012).
Campos, R. V., Lee, Y. C. & Drucker, D. J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134, 2156–2164 (1994).
Egan, J. M., Montrose-Rafizadeh, C., Wang, Y., Bernier, M. & Roth, J. Glucagon-like peptide-1 (7–36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology 135, 2070–2075 (1994).
Bullock, B. P., Heller, R. S. & Habener, J. F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137, 2968–2978 (1996).
Schlatter, P., Beglinger, C., Drewe, J. & Gutmann, H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul. Pept. 141, 120–128 (2007).
Wei, Y. & Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 358, 219–224 (1995).
Körner, M., Stöckli, M., Waser, B. & Reubi, J. C. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J. Nucl. Med. 48, 736–743 (2007).
Panjwani, N. et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology 154, 127–139 (2013).
Lennane, R. J., Carey, R. M., Goodwin, T. J. & Peart, W. S. A comparison of natriuresis after oral and intravenous sodium loading in sodium-depleted man: evidence for a gastrointestinal or portal monitor of sodium intake. Clin. Sci. Mol. Med. 49, 437–440 (1975).
Carey, R. M. Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ. Res. 43, 19–23 (1978).
Michell, A. R., Debnam, E. S. & Unwin, R. J. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu. Rev. Physiol. 70, 379–403 (2008).
Tang-Christensen, M. et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am. J. Physiol. 271, R848–R856 (1996).
Moreno, C., Mistry, M. & Roman, R. J. Renal effects of glucagon-like peptide in rats. Eur. J. Pharmacol. 434, 163–167 (2002).
Yu, M. et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J. Hypertens. 21, 1125–1135 (2003).
Crajoinas, R. O. et al. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am. J. Physiol. Renal Physiol. 301, F355–F363 (2011).
Gutzwiller, J.-P. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89, 3055–3061 (2004).
Girardi, A. C., Fukuda, L. E., Rossoni, L. V., Malnic, G. & Rebouças, N. A. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am. J. Physiol. Renal Physiol. 294, F414–F422 (2008).
Carraro-Lacroix, L. R., Malnic, G. & Girardi, A. C. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 297, F1647–F1655 (2009).
Hirata, K. et al. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem. Biophys. Res. Commun. 380, 44–49 (2009).
Pacheco, B. P. et al. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J. Hypertens. 29, 520–528 (2011).
Marina, A. S., Kutina, A. V. & Natochin, Y. V. Exenatide stimulates solute-free water clearance by the rat kidney in hyperhydration. Dokl. Biol. Sci. 437, 85–87 (2011).
Rieg, T. et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am. J. Physiol. Renal Physiol. 303, F963–F971 (2012).
Kim, M. et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 19, 567–575 (2013).
Skov, J. et al. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J. Clin. Endocrinol. Metab. 98, E664–E671 (2013).
Kutina, A. V., Marina, A. S., Shakhmatova, E. I. & Natochin, Y. V. Physiological mechanisms for the increase in renal solute-free water clearance by a glucagon-like peptide-1 mimetic. Clin. Exp. Pharmacol. Physiol. 40, 510–517 (2013).
Shakhmatova, E. I. et al. Exenatide stimulated solute-free water excretion by human kidney [Russian]. Ross. Fiziol. Zh. Im. I. M. Sechenova 98, 1021–1029 (2012).
Liu, Q. et al. The exenatide analogue AC3174 attenuates hypertension, insulin resistance, and renal dysfunction in Dahl salt-sensitive rats. Cardiovasc. Diabetol. 9, 32 (2010).
Park, C. W. et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J. Am. Soc. Nephrol. 18, 1227–1238 (2007).
Kodera, R. et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54, 965–978 (2011).
Hendarto, H. et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. 61, 1422–1434 (2012).
Ojima, A. et al. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am. J. Pathol. 182, 132–141 (2013).
Vaghasiya, J., Sheth, N., Bhalodia, Y. & Manek, R. Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul. Pept. 166, 48–54 (2011).
Liu, W. J. et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Ther. 340, 248–255 (2012).
Alter, M. L. et al. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press. Res. 36, 119–130 (2012).
Mega, C. et al. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp. Diabetes Res. 2011, 1–12 (2011).
Wang, Y. et al. Attenuation of renovascular damage in Zucker diabetic fatty rat by NWT-03, an egg protein hydrolysate with ACE- and DPP4-inhibitory Activity. PLoS ONE 7, e46781 (2012).
Robinson, L. E., Holt, T. A., Rees, K., Randeva, H. S. & O'Hare, J. P. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 3, e001986 (2013).
Monami, M., Dicembrini, I., Nardini, C., Fiordelli, I. & Mannucci, E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes. Metab. http://dx.doi.org/10.1111/dom.12175.
Vilsbøll, T., Christensen, M., Junker, A. E., Knop, F. K. & Gluud, L. L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344, d7771 (2012).
Kubota, A. et al. Pleiotropic effects of sitagliptin in the treatment of type 2 diabetes mellitus patients. J. Clin. Med. Res. 4, 309–313 (2012).
Ogawa, S. et al. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J. Exp. Med. 223, 133–135 (2011).
Mistry, G. C. et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J. Clin. Pharmacol. 48, 592–598 (2008).
Monami, M., Ahrén, B., Dicembrini, I. & Mannucci, E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 15, 112–120 (2013).
Von Eynatten, M., Gong, Y., Emser, A. & Woerle, H.-J. Efficacy and safety of linagliptin in type 2 diabetes subjects at high risk for renal and cardiovascular disease: a pooled analysis of six phase III clinical trials. Cardiovasc. Diabetol. 12, 60 (2013).
Bergenstal, R. M. et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 376, 431–439 (2010).
Pratley, R. E. et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 375, 1447–1456 (2010).
Dai, Y., Mehta, J. L. & Chen, M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-κB Activation. Cardiovasc. Drugs Ther. 27, 371–380 (2013).
Shah, Z. et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124, 2338–2349 (2011).
Van Genugten, R. E., Möller-Goede, D. L., van Raalte, D. H. & Diamant, M. Extra-pancreatic effects of incretin-based therapies: potential benefit for cardiovascular-risk management in type 2 diabetes. Diabetes Obes. Metab. 15, 593–606 (2013).
Gallwitz, B., Vaag, A., Falahati, A. & Madsbad, S. Adding liraglutide to oral antidiabetic drug therapy: onset of treatment effects over time. Int. J. Clin. Pract. 64, 267–276 (2010).
Jensen, E. P. et al. Activation of renal GLP-1 receptors located in the afferent arteriole causes an increase in renal blood flow. Diabetologia 56 (Suppl.), 255 (2013).
Thomson, S. C., Kashkouli, A. & Singh, P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am. J. Physiol. Renal Physiol. 304, F137–F144 (2013).
Deng, A. & Baylis, C. Locally produced EDRF controls preglomerular resistance and ultrafiltration coefficient. Am. J. Physiol. 264, F212–F215 (1993).
DeFronzo, R. A. et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr. Med. Res. Opin. 24, 2943–2952 (2008).
Ceriello, A. Oxidative stress and glycemic regulation. Metabolism 49, 27–29 (2000).
Bunck, M. C. et al. One-year treatment with exenatide vs. insulin glargine: effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis 212, 223–229 (2010).
Ishibashi, Y., Matsui, T., Takeuchi, M. & Yamagishi, S.-I. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 391, 1405–1408 (2010).
Ishibashi, Y., Matsui, T., Takeuchi, M. & Yamagishi, S. Sitagliptin augments protective effects of GLP-1 against advanced glycation end product receptor axis in endothelial cells. Horm. Metab. Res. 43, 731–734 (2011).
Puddu, A., Storace, D., Durante, A., Odetti, P. & Viviani, G. L. Glucagon-like peptide-1 counteracts the detrimental effects of advanced glycation end-products in the pancreatic β cell line HIT-T 15. Biochem. Biophys. Res. Commun. 398, 462–466 (2010).
Sakata, K. et al. Efficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product receptor for advanced glycation end product axis, and albuminuria in Japanese type 2 diabetes. Diabetes Metab. Res. Rev. 29, 624–630 (2013).
Chaudhuri, A. et al. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 97, 198–207 (2012).
Makdissi, A. et al. Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 97, 3333–3341 (2012).
Klonoff, D. C. et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 24, 275–286 (2008).
Monami, M., Marchionni, N. & Mannucci, E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur. J. Endocrinol. 160, 909–917 (2009).
Astrup, A. et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374, 1606–1616 (2009).
Esposito, K. et al. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 13, 594–603 (2011).
Monami, M., Lamanna, C., Desideri, C. M. & Mannucci, E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv. Ther. 29, 14–25 (2012).
Mentlein, R. Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul. Pept. 85, 9–24 (1999).
Marchetti, C. et al. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia 55, 236–244 (2012).
Hocher, B., Reichetzeder, C. & Alter, M. L. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press. Res. 36, 65–84 (2012).
Tagore, D. M. et al. Peptidase substrates via global peptide profiling. Nat. Chem. Biol. 5, 23–25 (2009).
Kiemer, A. K., Fürst, R. & Vollmar, A. M. Vasoprotective actions of the atrial natriuretic peptide. Curr. Med. Chem. Cardiovasc. Hematol. Agents 3, 11–21 (2005).
DeFelice, A. F. & Brousseau, A. Natriuretic and vasodilating activities of intrarenally administered atriopeptin II, substance P and bradykinin in the dog. J. Pharmacol. Exp. Ther. 246, 183–188 (1988).
Anderson, J. V., Struthers, A. D., Payne, N. N., Slater, J. D. & Bloom, S. R. Atrial natriuretic peptide inhibits the aldosterone response to angiotensin II in man. Clin. Sci. (Lond.). 70, 507–512 (1986).
Imaizumi, T. & Takeshita, A. Influence of ANP on sympathetic nerve activity and chronotropic regulation of the heart. J. Cardiovasc. Electrophysiol. 4, 719–729 (1993).
Minson, R., McRitchie, R. & Chalmers, J. Effects of neuropeptide Y on the renal, mesenteric and hindlimb vascular beds of the conscious rabbit. J. Auton. Nerv. Syst. 27, 139–146 (1989).
Tögel, F., Isaac, J., Hu, Z., Weiss, K. & Westenfelder, C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 67, 1772–1784 (2005).
Zaruba, M.-M. et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell 4, 313–323 (2009).
Hocher, B., Sharkovska, Y., Mark, M., Klein, T. & Pfab, T. The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int. J. Cardiol. 167, 87–93 (2013).
Fadini, G. P. et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1α. Diabetes Care 33, 1607–1609 (2010).
Gilbey, M. P., McKenna, K. E. & Schramm, L. P. Effects of substance P on sympathetic preganglionic neurones. Neurosci. Lett. 41, 157–159 (1983).
O'Connor, T. M. et al. The role of substance P in inflammatory disease. J. Cell. Physiol. 201, 167–180 (2004).
Wu, H. et al. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. 21, 1878–1890 (2010).
Kim, J., Sohn, E., Kim, C.-S., Jo, K. & Kim, J. S. The role of high-mobility group box-1 protein in the development of diabetic nephropathy. Am. J. Nephrol. 33, 524–529 (2011).
Hattori, S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr. J. 58, 69–73 (2011).
Groop, P.-H. et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 36, 3460–3468 (2013).
Zhang, H., Zhang, X., Hu, C. & Lu, W. Exenatide reduces urinary transforming growth factor-β1 and type IV collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press. Res. 35, 483–488 (2012).
US Department of Health and Human Services. Guidance for Industry: diabetes mellitus— evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. [online], (2008).
White, W. B. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013).
Pendergrass, M., Fenton, C., Haffner, S. M. & Chen, W. Exenatide and sitagliptin are not associated with increased risk of acute renal failure: a retrospective claims analysis. Diabetes Obes. Metab. 14, 596–600 (2012).
Tofovic, D. S., Bilan, V. P. & Jackson, E. K. Sitagliptin augments angiotensin II-induced renal vasoconstriction in kidneys from rats with metabolic syndrome. Clin. Exp. Pharmacol. Physiol. 37, 689–691 (2010).
Jackson, E. K. & Mi, Z. Sitagliptin augments sympathetic enhancement of the renovascular effects of angiotensin II in genetic hypertension. Hypertension 51, 1637–1642 (2008).
Marney, A., Kunchakarra, S., Byrne, L. & Brown, N. J. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension 56, 728–733 (2010).
Jackson, E. K. Dipeptidyl peptidase IV inhibition alters the hemodynamic response to angiotensin-converting enzyme inhibition in humans with the metabolic syndrome. Hypertension 56, 581–583 (2010).
Chaykovska, L. et al. Effects of telmisartan and linagliptin when used in combination on blood pressure and oxidative stress in rats with 2-kidney-1-clip hypertension. J. Hypertens. 31, 2290–2299 (2013).
Ishibashi, Y. et al. Glucagon-like peptide-1 inhibits angiotensin II-induced mesangial cell damage via protein kinase A. Microvasc. Res. 84, 395–398 (2012).
Mima, A. et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 61, 2967–2979 (2012).
Butler, P. C., Elashoff, M., Elashoff, R. & Gale, E. A. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 36, 2118–2125 (2013).
Elashoff, M., Matveyenko, A. V., Gier, B., Elashoff, R. & Butler, P. C. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141, 150–156 (2011).
Drucker, D. J. Incretin action in the pancreas: Potential promise, possible perils, and pathological pitfalls. Diabetes 62, 3316–3323 (2013).
Nauck, M. A. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care 36, 2126–2132 (2013).
SAFEGUARD safety evaluation of adverse reactions in diabetes. Safeguard-diabetes.org [online], (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Author information
Authors and Affiliations
Contributions
M. Diamant, M. H. A. Muskiet and M. M. Smits researched the data for the article, made a substantial contribution to discussions of the content, wrote the article, and reviewed and/or edited the manuscript before submission. L. M. Morsink made a substantial contribution to discussions of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. Diamant is a consultant for Abbott, Astra Zeneca, Boehringer–Ingelheim, Bristol-Myers Squibb, Eli Lilly, GI Dynamics, Merck Sharp & Dohme, Novo Nordisk, Poxel Pharma and Sanofi. She is also a speaker for Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, Novo Nordisk and Sanofi. Through M. Diamant, the VU University Medical Centre receives research grants from Abbott, Astra Zeneca, Boehringer–Ingelheim, Bristol-Myers Squibb, Eli Lilly, Medtronic, Merck Sharp & Dohme, Novo Nordisk and Sanofi. M. Diamant receives no personal payments in connection to the above-mentioned activities: all funds are directly transferred to the Diabetes Centre's nonprofit Research Foundation. The other authors declare no competing interests.
Supplementary information
Supplementary Table 1
Evidence of renoprotective effects of established glucose lowering agents in patients with T2DM (DOC 61 kb)
Supplementary Table 2
Evidence of renoprotective effects of established blood pressure lowering agents in patients with T2DM (DOC 116 kb)
Supplementary Table 3
Evidence of renoprotective effects of an established multifactorial intervention in patients with T2DM (DOC 41 kb)
Supplementary Table 4
Renal effects of GLP-1R agonists and DPP-4 inhibitors in preclinical studies (DOC 75 kb)
Rights and permissions
About this article
Cite this article
Muskiet, M., Smits, M., Morsink, L. et al. The gut–renal axis: do incretin-based agents confer renoprotection in diabetes?. Nat Rev Nephrol 10, 88–103 (2014). https://doi.org/10.1038/nrneph.2013.272
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.272
This article is cited by
-
Characterization of gut microbiota in patients with stage 3–4 chronic kidney disease: a retrospective cohort study
International Urology and Nephrology (2023)
-
Mechanisms and efficacy of traditional Chinese herb monomers in diabetic kidney disease
International Urology and Nephrology (2023)
-
Efficacy and safety of sitagliptin treatment in older adults with moderately controlled type 2 diabetes: the STREAM study
Scientific Reports (2023)
-
Pan-Src kinase inhibitor treatment attenuates diabetic kidney injury via inhibition of Fyn kinase-mediated endoplasmic reticulum stress
Experimental & Molecular Medicine (2022)
-
The combination of soluble tumor necrosis factor receptor type 1 and fibroblast growth factor 21 exhibits better prediction of renal outcomes in patients with type 2 diabetes mellitus
Journal of Endocrinological Investigation (2021)